
[Editor's note] American chemist K. Barry Sharpless shared the 2022 Nobel Prize in Chemistry with two other chemists. This is the second time that Sharpless has won the Nobel Prize in Chemistry. The last time was 21 years ago in 2001.
On the afternoon of October 5, 2022, after the Nobel Prize in Chemistry was announced, The Paper contacted Dr. Dong Jiajia, who had been engaged in postdoctoral research in Sharpless's laboratory, to interpret the relevant achievements of the award. Dr. Jiajia Dong is currently a tenured professor at the School of Translational Medicine, Shanghai Jiao Tong University. He translated and sorted out Dr. Sharpless's speech at the 2019 Priestley Medal Award Ceremony, which The Paper is authorized to repost.
After reading Kevin Kelly's "Out of Control" together, I discussed it on a beach walk with MG, and our favorite part was what Kelly called "God's Game (the process of creation, a metaphor for evolution)" that paragraph. Kelly said, “The irony of God’s process of creation (evolution) is that choosing to let go (not to control and intervene) is the only way to win.” Later, this inspired us.

The principle of the Priestley Medal is to reward "those who have contributed to the discipline of chemistry", but my contribution is really limited to chemists: to help you achieve more reliable ways to synthesize chemical bonds, (Build reliable portals in the vast molecular world) to achieve molecular functions. As an ordinary researcher with a less common research method, I can stand here tonight, and I am very grateful and very emotional.

Professor Karl Barry Sharpless (2019)
Hopefully today's talk will also be a contribution (to chemists) of mine. I was born into a not-so-perfect middle-class family. I was born and went to school in Philadelphia, where my father was a surgeon who worked in Philadelphia. But if someone asks me, "Where is your home?" I don't hesitate to answer, "Manasquan, NJ!"[1]

Karl Barry Sharpless with his mother
My mother grew up there, and every weekend or summer she would take my sister and me to the beach. On a wooded bluff by the Manasquan River, my parents bought a prefab cabin of the kind made by Sears Roebuck—four tiny bedrooms with living room and kitchen in between, my mother She and her friends often meet there with their children. My mother is very happy when there are lots of people, but she's a bit bipolar and sometimes can't handle me, and my father doesn't have time for me on weekends.
Because my parents did not discipline me strictly, it can be said to be indulgent, I have been looking for the surprises and excitement I need very casually since I was a child. My self-study and growth began when I was six years old, when I found an 8-foot dinghy with a motor, and drove the dinghy up the Manasquan River secretly and on my own, and drove for a few miles, It even drove past the estuary and went directly to the sea. When I was in elementary school, purely by my own interests, I had become a qualified sailor, a fisherman capable of catching eels, crabs, and a keen and inquisitive observer and experimentalist. Finally, before I graduated from elementary school, I was able to support myself and become a fearless self-learner.
In fact, when my parents first met my wife, Jan Sharpless, my mother said to her, "Good luck, I've never managed to teach this guy anything."

Karl Barry Sharpless and Jan Sharpless (Switzerland, 1987)
When I was 14, I was the youngest guy on a fishing charter at Brill Dock. We have to do everything we can to make our boat rental customers happy, and to take pictures of the harvest on the deck at the end of the day so that we can publicize and attract more customers. The competitive fishing life makes one decisive, and the daily maintenance of the boat requires complete conscientiousness. In order to get more tips from guests, for me, I have to overcome my shy side and disguise myself.

Karl Barry Sharpless (15, left) fishing with Uncle Dink on the boat

Karl Barry Sharpless (24)
Middle school life didn't interest me too much either - I was just a not-so-bad student. I would go with my father when he went to the bookstore to buy his medical books, and then I would choose some books on chemistry or maritime studies. I still remember how excited I was when I first learned about evolution through Darwin's The Voyage of the Beagle, but my favorite book was on steroid biosynthesis. When I saw that the three methyl groups of lanosterol actually disappeared and turned into carbon dioxide, I said, "Amazing!" This is a great primer on the oxidation process.
Maybe everyone is different, but I am really different. But I really don't want my students to experience these "differences". However, I have learned some experience in scientific research that can benefit everyone, so I will open my personal "suitcase" next, and I hope you will find it helpful after listening.
The legendary Swiss chemist Albert Eschenmoser said of me: "When talking to Barry, it's totally unpredictable what he's going to say. With ordinary people, you can Predict the content of your conversations, but not with him."
The non-linearity of thinking (jump thinking) is probably my most obvious, confusing, and even annoying characteristic. I like the definition of non-linear thinking: "Humans think in multiple directions at once, not just in one "logical" direction." At the same time, I need something exciting, exciting, even scary, inspiration And unusual - these elements make me feel my presence.
At Dartmouth College, I got an F in my first English essay, which was a shock to me. Fear has turned me into an avid learner, and I study hard until no problem can overwhelm me. Only chemistry is relatively easy for me. I have inherited my father's unforgettable memory, and I have relied on this to learn those chemical reactions all my life, so natural science courses are relatively easy for me, and I can also have time to go Master the skills to be a good student in the classroom. The fear of failure kept me going, but the wonderful teachers and the joy of learning really transformed me into a good scholar, especially a lover of literature. I like the "inevitable visual cognition" that James Joyce [2] mentioned in Ulysses.
I was a pre-medicine student, but I joined Assistant Professor Tom Spencer's lab full of arrows on electron transfer mechanisms. A chemist knows what life is like, and once I became interested in solving his chemistry problems, I recklessly prepared myself for experiments by teaching myself all the compounds I could find, mostly by smell. In our spare time, we discussed chemistry by drawing molecular structures on the blackboard, which became one of my greatest hobbies. At the same time, I also started to fall in love with the periodic table.
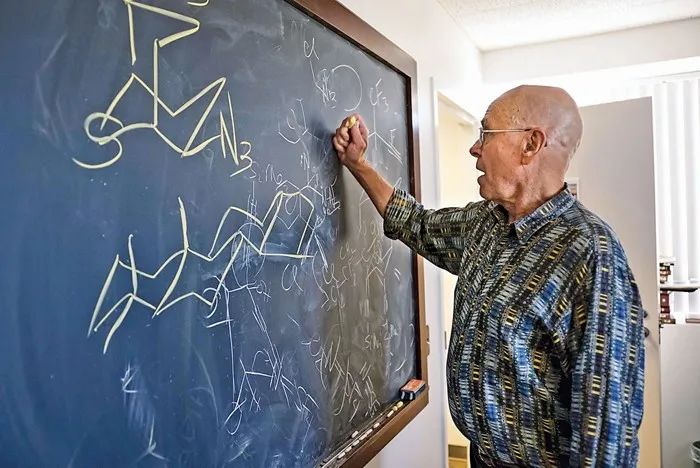
Barry loves writing on the blackboard to discuss chemistry in his office (Scripps Research)
Since I never stayed in school during the summer, I didn't have the opportunity to further expand my research program, so Tom Spencer (the man I'm most grateful for) persuaded me to give up medical school and try a year in the graduate department of the School of Chemistry . He recommended me to his advisor, Professor Eugene van Tamerlen of Stanford University [4].

Professor Eugene E. van Tamelen (1925-2009)
My research topic was the selective oxidation of polyenes, but I failed the physical organic chemistry exam (once every six months) , so I almost cried and read books for six months in the chemical library for the topic . But when I went back to the lab, I had enough knowledge for daily discussions with postdocs, especially Professor Tamerlen.
I was fortunate enough to be placed with Bob Coates, then a postdoc in the lab, who is now emeritusly retired from the University of Illinois at Urbana-Champaign. Under his careful guidance, I learned my first "research trick": If you can falsify the "seemingly correct" conclusions of the early experiments quickly enough, the faster you will move forward and find the next new one. 's discovery. I call this method: "falsification method". Later, when my students bring good experimental results, I will definitely say: "Go and let this reaction fail! (Find the limitations of reactivity) Design several different ideas while challenging reactivity, quickly Advance those ideas that survive, always looking for reactive cues and devising different ways to question them.
Like my parents, MIT treats their assistant professors very indulgently, allowing a professor like me who fears and hates to write to not apply for NIH research grants after spending two years without applying for grants. Days later, department chair Glenn Berchtold gave me two options, either jump into the sea and go on a treasure hunt on the Andrea Doria wreck, or write a grant application Book.
The year I wrote the grant application really changed my life. The knowledge I learned from reading a lot in the Stanford library gave me inspiration. At MIT, I began to seriously search for those elements in the periodic table whose reactivity was not yet studied. Those rigorous and detailed chemical records before the mid-20th century allowed me to really begin to understand and compare the properties of molecules, including their color, shape, solubility, and stability.
The strange properties of selenium have fascinated me since my early days at Dartmouth, in part because it is an essential element of life and has redox activity. At MIT, what I had learned from a lot of related reading at Stanford in my early years became subconscious, which led me to look at the mechanism of SeO2 chemistry that has been published. In fact, the previous mechanism was not possible, so we investigated it in the laboratory. Amazingly, the field was completely open to us; I found my way. Next, we quickly made a number of important contributions. Selenium chemistry became the subject of my NIH grant application, and I quickly became known.
Our lab is just getting started, and our approach is to explore those gaps in the periodic table that lack literature. We have greatly expanded the scope of synthetic chemistry by discovering many useful alkene oxidation reactions by taking advantage of "terrifying dark places" (biases and phobias) that no other chemist wishes to explore.
In hindsight, it is surprising that the effect of simple "biases and phobias" in the field of chemistry was so great that my lab's research direction: removing "phobias" from the periodic table, having "exclusivity" for many years.
Presumably because working on the Manasquan River, IQ seemed less important, so my later ambitions were never drawn to the game of complexity - thinking that complex synthetic chemistry was the only worthy direction. That's how I became a process chemist by accident, which is blue collar by pharmaceutical company standards because the medicinal chemists there are elite.

Karl Barry Sharpless visiting a chemical plant in Texas (1983)
For me, writing is as painful as filling my teeth without an anaesthetic, so I've never published anything that doesn't make much sense, probably only Hans Reich at the University of Wisconsin and I can compete on this (Referring to not like to post articles). The pressure that I need to publish more important results is removed, and in the process, taking the time to document and flesh out them, gives us more and more interesting opportunities (referring to the creation of new idea). The new methods we published at the time always described at least one unprecedented selective transformation of complex natural products, always involving very detailed experimental procedures.
By the mid-1970s, our method had received a lot of applications and citations, which gave us a lot of encouragement, which accelerated our enthusiasm and creativity for work in this field (olefin oxidation). The important discovery of my postdoc, the late Bob Michaelson in 1973, and our group's love of olefin oxidation chemistry, led directly to my postdoc Tsutomu Katsuki's discovery in 1980 Asymmetric epoxidation (AE) reaction [6].

The famous Sharpless asymmetric epoxidation

Katsuki's experimental record when he discovered the optimal conditions for epoxidation (photographed by Dong Jia's family in 2015)
Also in 1980, graduate student Steve Hentges discovered the perfect stoichiometric asymmetric osmylation reaction, which gave me confidence in the discovery of more asymmetric catalytic reactions, despite the thought that was impossible.
After discovering asymmetric epoxidation, I needed new stimuli, so I was ready to look into new territory.
About 2 years after the discovery of asymmetric epoxidation, I had a life-changing conversation with the eminent organic chemist Sir Derek Harold Richard Barton [7]. When I told him that I was going to stop doing asymmetric catalysis but to break new ground, he asked me sternly, "Do you think there are other catalytic asymmetric reactions that need to be discovered?" I said, "Yes. Yes, I know there is." Sir Derek said, if I didn't, the asymmetric epoxidation could be an orphan reaction, and Derek Barton is a convincing one!
The asymmetric epoxidation reaction keeps us so busy that we don't go back to Hunters' findings, but this direction was developed by my postdoc Eric Jacobsen and the late Istvan Marko ( Istvan Marko led yet another discovery of gold: asymmetrically catalyzed bishydroxylation (AD) [8], a real victory, an excellent, general, and highly practical reaction.

Famous Sharpless Asymmetric Dihydroxylation
After discovering asymmetrically catalyzed bishydroxylation, I was finally able to move on (change research direction), but my team wasn't ready. I had a lot of very talented postdocs in my lab at the time who came to study asymmetric catalysis, but my communication skills and leadership let me down. How can I free them from ingrained beliefs, break out of the "religion" I've created, and challenge new things? Lab morale dropped badly, and later, when I turned everyone's direction to click chemistry, the lab was nearly empty (people were gone).
I don't have any good advice on lab management, but advice on scientific discovery is something else: I'm always on the lookout for clues, and always follow those good ones, even at the expense of the lab's current priorities. cost. Don't worry about what you'll miss, because in the end the only thing that matters as a researcher is what you find, because there's a lot of new stuff waiting to be discovered. Asking good questions is crucial as a researcher. Parallel Hypothesis Inference [9], a large number of parallel feasible hypotheses, and the periodic table of elements, will never let you down.
In 1982, I wrote an NIH grant application proposal, hoping to use the reaction of amines and epoxides to link those unnatural molecular modules together, and then use combinatorial chemistry to synthesize them, hoping that the products will have interesting molecules. Function. NIH did not support us.
(1992) I joined the Scripps Research Institute in California and started consulting for a combinatorial chemistry company. At the same time, I began to focus on finding a way to quickly and reliably discover new chemical reactivities and molecular functions. My plan at the time was to use only a few optimal reactions to link molecular modules, hoping to perform quantitative reactions without solvent (and thus achieve molecular function quickly). My student Janet Elizabeth Pease tried what we thought were the best six reactions at the time in 1996 or 1997 with 96% yields, and these became the first attempts at click chemistry. But then we quickly abandoned solvent-free reactions in favor of sticking with water as the solvent.
Among the many candidates, Jan and I decided to call this approach "click chemistry." Because in our opinion it best describes what we think, the metaphor of the seat belt in the rear seat of the car best explains our goal: only the predetermined buckle can connect to the corresponding interface - the middle seat belt The side seat belts cannot be buckled. The success of the connection is guaranteed and once established is permanent. In a car, the spring-locked snaps make a clicking sound. [10]
My colleague Hartmuth Kolb at the Scripps Research Institute, who has been the engine driving click chemistry since the beginning; at the National Meeting of the American Chemical Society in the spring of 1999 , our first public lecture on the subject was: "Click Chemistry, A Chemical Concept for Merging Processes and Discoveries." My former graduate student (1980s, MIT) MG Finn later joined SK Lipps Department of Chemistry, this is a big deal for me. He quickly joined us, like a philosopher, who brought a logical foundation to click chemistry. Finn articulates concepts that Hartems and I have always wanted to articulate.
We call ourselves the "three friends" [11] and call our click chemistry manuscript the "manifesto". The paper was submitted to the German Journal of Applied Chemistry in August 2000. The journal's editor-in-chief, Peter Gölitz, was willing to push back on negative reviews, but he did worry about whether I would face the consequences and become a "fool" in the international chemistry community. In May 2001, "Click Chemistry: Achieving Molecular Function Through A Few Good Reactions" was published online. [12]

The click chemistry paper that was rejected by all the judges is also the most cited paper in the field of synthetic chemistry.
So much happened that year (2001): I won many awards, including the Nobel Prize in Chemistry [13], and my 60th birthday celebration with 33 former Sharpless group members in San Diego Spring USA Presented at the National Meeting of the Chemical Society. It was at the banquet of that conference that I received the best gift of my life! When the famous MIT George Büchi died, his younger colleague (and former student of mine) Greg Fu inherited Büchi's library. Greg gave me part of it: maybe a ton of Houben-Weyl Methoden der Organischen Chemie [14] (including all volumes, 1909-1986, which even cites 1834!). The series that Greg gave me represents a golden age in the history of human chemical discovery - no chemist could have hoped for more!

Professor Sharpless won the 2001 Nobel Prize in Chemistry for his discovery of asymmetric catalytic oxidation reactions

Professor Sharpless's 2001 Nobel Prize in Chemistry

Professor Sharpless and his Houben-Weyl series (almost every book is full of his notes!)
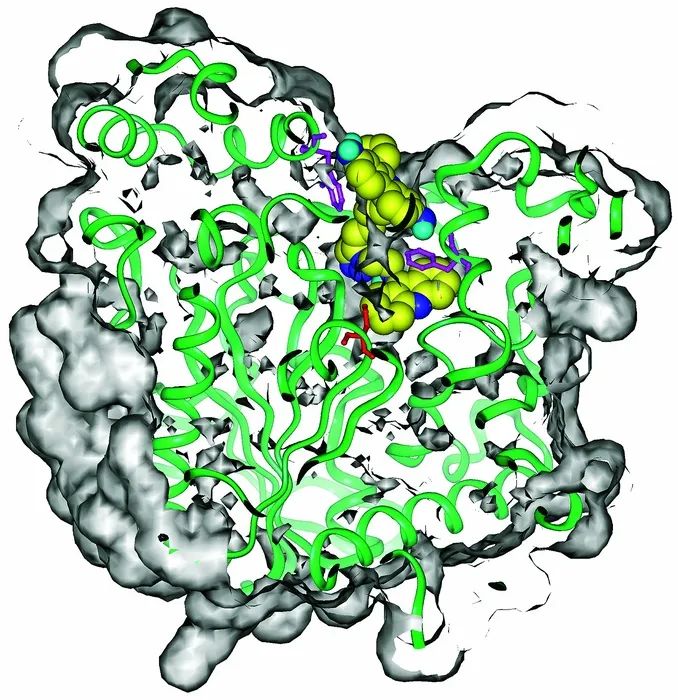
Previously, after reading Kevin Kelly's "Out of Control"[15] together, I discussed with MG on a beach walk, and our favorite part was what Kelly called "God's Game (Creation). process, a metaphor for evolution)”. Kelly said, "The irony of God's process of creation (evolution) is that choosing to let go (not to control and intervene) is the only way to win." Later, this inspired us to have the two reactants in the The binding site of a well-known enzyme reacts passively and selectively [16]. Of the 98 possible reaction partners, the picky acetylcholinesterase chose to make only one inhibitor, but this inhibitor was much more active than any of the others, and in situ click chemistry was born. This work was done in collaboration with Professor Palmer Taylor of the University of California, San Diego.

in situ click chemistry
My postdoc Luke Green's work on this enzymatic reaction led directly to the discovery of CuAAC (copper-catalyzed cycloaddition of azides to alkynes). Albert Eschenmoser [17] gave this work high marks, saying that the discovery of CuAAC "is such a large improvement in the efficiency and substrate range of known reactions that it clearly belongs to the The most important discovery in chemistry." We published both studies in 2002 (referring to In situ click chemistry, CuAAC.)

CuAAC (Copper-catalyzed cycloaddition of azides to terminal alkynes)
Several companies based on click chemistry have emerged, the most fascinating of which is Olaplex. They do hair restoration through click chemistry (in this case the thiol-double bond click chemistry), Kim Kardashian can change her hair color every few days and her hair still looks Hydrated.
In 2002, I had the privilege of being invited to the first ever "Comforth Lecture" at the University of Sydney with Craig Hawker, a distinguished materials scientist at the University of California, Santa Barbara, who Started several businesses including Olaplex. He wrote to me: "The inspiration for Olaplex goes back to your speech in Sydney many years ago, which completely changed my thinking. As you mentioned, Sir John Warcup Cornforth[18] ) in my own words: 'It is useless to offer so-called elegant, difficult and expensive processes to industrial chemists whose ideals are those reactions that can even be reworked in a discarded bathtub. Only one without One-armed disabled workers of the culture can then be proficient in the process of continuously collecting reaction products in 100% purity and yield directly through drain holes.' Your key information about reaction efficiency, simplicity and orthogonality (these are all Click on the chemistry logo) resonates even more in the chemistry community today."
Another disruptive technology in the field of click chemistry was developed by MG Finn in 2003. Biological scientists now use kits based on this technology every day, but they may not even know they are using click chemistry.

Sir John Cornforth's Quotes on Process Chemistry
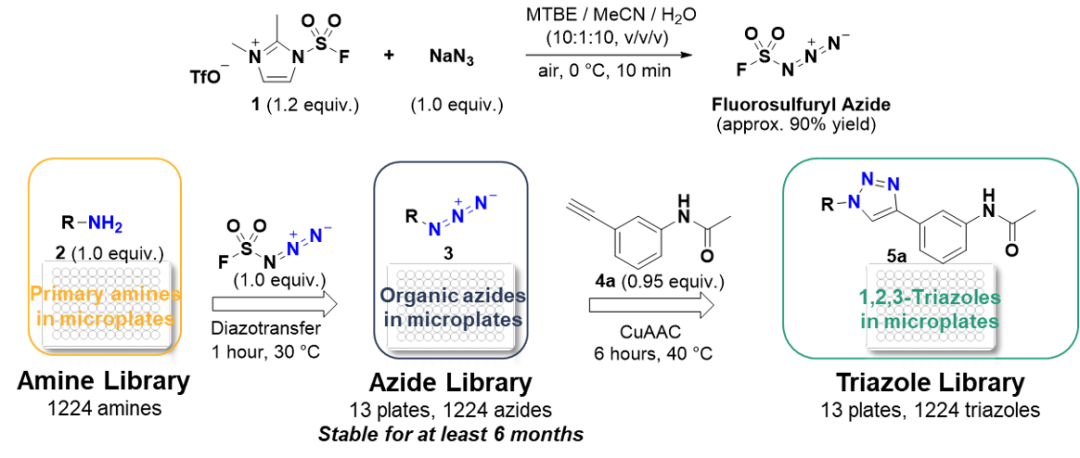
My research group has been hoping to repeat the experiments of the German chemist Wilhelm Steinkopf (on the synthetic chemistry of sulfonyl fluorides) from 1927 to 1930, but it has been unsuccessful for many years. My postdoc Jiajia Dong didn't give up, and after receiving a bottle of sulfuryl fluoride from Dow, he finally made it. His discovery opened the hexavalent sulfur-fluorine exchange reaction (SuFEx), the second near-perfect click chemistry reaction discovered in our laboratory. SuFEx unexpectedly opens up a whole world of molecules linked via sulfates. He is now working at the Shanghai Institute of Organic Chemistry.

Discovery of the hexavalent sulfur-fluorine exchange (SuFEx) reaction

Professor Sharpless and Dong Jiajia (taken in 2015, Scripps Research)
Now, Dong Jia's lab has discovered another brand-new, near-perfect click chemistry reaction [19]. I predict that with these four near-perfect click reactions (mentioned above), chemists will construct endless chemical space possibilities from the bottom up through completely predictable, precise synthesis.

Discovery of efficient diazo transfer reactions of primary amines by FSO2N3
So many people who have worked with me have helped me get to where I am today, and I am grateful for all of them. I also want to apologize to the many colleagues who have worked in my lab, some of whom are not getting the treatment they deserve, the articles they deserve, or even much opportunity to talk to me. I would also like to say sorry to my colleagues in the chemical industry who have never had time to thank me, who have given me their work, gifts and favors. sorry! Actually, about a lot of things, I was a complete failure. Over the years, I have enjoyed being a chemist completely selfishly, and a lot of people have made sacrifices for it. I really appreciate you guys and every molecule in me thanks you!
I'm really happy to see "Simple (Chemistry)" and "(Molecular) Function" being embraced in my lifetime. In closing, I would like to quote a letter written to me in 2015 by Stephen Buchwald of the Massachusetts Institute of Technology, reminding me that it takes courage to break through the mediocrity in science and reach no man's land , which really comforts me. He is probably my only follower of chemists of the last century.
"Your achievements in chemistry have had a profound impact on the direction of my research - your focus on simple and useful chemistry. Your help has been the key to my success. Over the years, whenever I have a problem, I have Turning to this principle, which is: '(same problem) What would Barry do?' Along the way, I have been faithful to your research philosophy of 'do important and practical chemistry', which has made me who I am today . As I approach my 60th birthday, I just want to thank you."
Translator press:
[1] A seaside town, New Jersey, USA.
[2] Irish Modernist Writer
[3] Tom Spencer, Dartmouth College, renowned bioorganic chemist.
[4], Eugene E. van Tamelen, Stanford University, bioorganic chemist, pioneer in the field of biomimetic synthesis.
[5] Phobia is a type of anxiety disorder, which is characterized by persistent fear and fear of certain things or situations. Barry especially likes to use the word to describe some chemists' unscientific, popular perception of something that leads to prejudice and ignorance.

[6] The Sharpless asymmetric epoxidation reaction (AE reaction) is an asymmetrically selected chemical reaction that can be used to prepare 2,3-epoxy alcohols from primary or secondary allyl alcohols. It is an asymmetric epoxidation reaction named after the principal inventors, Barry Sharpless and Xiang Yuexu.
[7] Sir Derek Harold Richard Barton, master of organic chemistry, winner of the 1969 Nobel Prize in Chemistry.

[8] Sharpless asymmetric dihydroxylation, often directly referred to as asymmetric dihydroxylation (AD reaction), is the basis of Barry Sharpless's Upjohn dihydroxylation. Asymmetric dihydroxylation of alkenes catalyzed by cinchonadine derivatives, discovered in 1987. Like the Sharpless epoxidation, this reaction is one of the most important reactions in modern organic synthesis.
[9] Strong inference, is a scientific research (logical thinking) methodology that emphasizes parallel assumptions rather than single causal inference: "In philosophy of science, strong inference is a model of scientific inquiry that emphasizes the need for alternative hypotheses, rather than a single hypothesis to avoid confirmation bias.”


[11] This is a famous American comedy film, Three Amigos.
[12] After the article was submitted, the three heavyweight judges unanimously requested the journal to reject the manuscript, and Peter Gritz, the legendary editor-in-chief of applied chemistry in Germany, forcefully published the article, which now ranks among the highest in German applications in citations. The first in the history of all articles published in a chemistry journal, and Pete Gleiz also listed this article as his favorite. The legendary level of this story is extremely rare in the history of science. Original by Peter in C&EN (excerpt):
“What was your favorite paper that Angewandte published during your tenure?”
“There is a famous click chemistry paper by Barry Sharpless that we published in 2001. There was a lot of skepticism that this was not new, nor was it needed. I overruled that advice, and I think click chemistry has changed many fields, from chemical biology to materials science.”
https://cen.acs.org/articles/95/i48/German-journal-became-top-tier.html
[13] Barry Sharpless shared the 2001 Nobel Prize in Chemistry for his work on asymmetric oxidation. He also shared the award with: William Standish Knowles and Ryoji Noyori.
[14] The famous synthetic chemistry series, which was renamed Science of Synthesis in 2000.
[15] Out of Control: The New Biology of Machines, Social Systems, and the Economic World is a 1994 book by Kevin Kelly. Out of control topics include cybernetics, emergence, self-organization, complex systems, and chaos theory, and this book can also be considered a work of techno-utopianism. Runaway is also one of three books that the Wachowski brothers asked Keanu Reeves, who played Neo, to read before he started reading the script (the other two being "The Matrix" in 1999). Imitation and Simulation" and "Evolutionary Psychology")


Barry and Penguin
[16] Here they let the protein first connect with the weakly binding small molecule building block containing azide functional group, and use the protein pocket as the "container" for the reaction, and directly add a variety of different structures containing terminal alkyne functional group into the system. Molecular building blocks, then based on the orthogonal chemical reactivity of terminal alkynes with azide 3+2 cycloadditions, induced by the microstructure within the pocket, the protein synthesized one of the strongest inhibitors against itself with extreme selectivity The inhibitor molecule inhibits acetylcholinesterase with a dissociation constant value (Kd) of 77fM, in situ click chemistry, no design, and the protein chooses to synthesize its own inhibitor! An era has begun, and click chemistry is ready to take off! The discovery of the CuAAC reaction was precisely because of this discovery that Barry's group had to find a way to selectively synthesize 1,5 triazole, and then everything that followed was a great history...
[17] Albert Eschenmoser, Swiss scientist, master of organic synthesis, famous for synthesizing vitamin B12.
[18] Sir John Warcup Cornforth, 1975 Nobel Prize in Chemistry for the stereochemistry of enzyme-catalyzed reactions.
[19] Here Barry refers to the high-efficiency diazo transfer reaction of FSO2N3 for primary amines. It is worth noting that this reaction was not published or even submitted at the time. We only voted for the first two months after Barry's speech. The paper was officially published in the British journal Nature in October of that year.
Modular point-and-click compound library construction https://doi.org/10.1038/s41586-019-1589-1
[20] Stephen L. Buchwald, famous organic chemist, professor at the Massachusetts Institute of Technology. Known for developing transition metal catalyzed reactions.


appendix:
The Priestley Medal is the highest award awarded by the American Chemical Society and is currently selected annually to encourage scientists who have made outstanding contributions to the field of chemistry. Established in 1922, the prize is named after British chemist Joseph Priestley. The selection was held every three years until 1944.
To recognize distinguished services to chemistry. (ACS)
Original article first published on C&EN, March 31, 2019 | APPEARED IN VOLUME97, ISSUE 13
Priestley Medal address 2019: A simple life—finding function and making connections
Original link: https://cen.acs.org/people/awards/Priestley-Medal-address-2019-simple-life-finding-function-and-making-connections/97/i13
(Original title "A Simple Life - Finding Molecular Functions and Links (Speech at the 2019 Priestley Medal)")


