[Editor's Note] Funded by the Shanghai Municipal Science and Technology Commission (Project No.: 22DZ2304300), The Paper reported on the popularization of results awarded with national and Shanghai municipal science and technology awards in collaboration with World Science.
This article focuses on the 2021 Shanghai Science and Technology Progress First Prize project "Creation of a Disease Classification Model for Intestinal Microbiota and Clinical Application of Microbiota Transplantation," led by Professor Qin Huanlong from the Tenth People's Hospital affiliated with Tongji University.
As society ages and the pressure on younger individuals increases, the types and severity of intestinal diseases are undergoing significant changes: there is a rise in drug-intervention-related intestinal lesions, and there has been a notable increase in chronic diseases combined with gastrointestinal problems, with the proportion of individuals over 70 with co-existing gastrointestinal diseases reaching as high as 32%. Extraintestinal conditions such as Alzheimer’s disease, Parkinson’s disease, and depression are found in up to 90% of patients facing constipation. New "incurable diseases," such as autism and inflammatory bowel disease, are increasingly prevalent among intestinal disorders. Unfortunately, traditional drug treatments have not promptly adapted to the evolving spectrum of intestinal diseases in modern society, resulting in a large number of treatment-resistant patients emerging. Furthermore, the use of antibiotics and other modern drugs has led to the emergence of new intestinal disorders (such as immune-mediated enteritis). Therefore, there is an urgent need to explore new treatment approaches that are safe, effective, affordable, and easy to implement.
The Gut Microbiome Theory Leading the Transformation of Medical Technology in the 21st Century
As early as the 3rd century BC, Hippocrates, the father of modern medicine, proposed the scientific concept that “all diseases begin in the gut.” Recent research shows that the vast majority of chronic diseases, including constipation, diarrhea, obesity, depression, fatty liver, autism, cardiovascular diseases, and Alzheimer’s disease, are associated with dysbiosis of the gut microbiota. The gut microbiota participates in the host’s dietary metabolism and produces various regulatory molecules, such as short-chain fatty acids, bile acids, and hormones, which regulate multiple organs, affecting nutrient absorption, immune response, fat storage, brain excitability, and blood sugar levels. When the intestinal mucosal barrier is compromised, the gut microbiota can translocate to distant organs causing localized inflammation and participating in the distal metastasis of intestinal tumors. Hence, uncovering the essential relationship between the gut microbiota and diseases, and improving disease symptoms by restoring the healthy homeostasis of the gut microbiota has become a revolutionary strategy for clinical treatment in the 21st century.
In fact, as early as the Eastern Jin Dynasty in ancient China, Ge Hong’s text “Emergency Prescriptions from the Golden Cabinet” detailed the treatment of typhoid with intestinal microbiota, stating, “If the fever has reached its peak for six or seven days, and if the patient experiences severe discomfort in the chest and abdomen, mad talk of seeing ghosts, and a desire to get up and run: extract fecal juice, and drink for several cups until one or two liters; this is called Huanglong Decoction, and it is better if aged.” However, the large-scale clinical practice of modern medicine targeting gut microbiota to treat diseases has only begun in the last decade. This therapy is referred to as "Fecal Microbiota Transplantation" (FMT), which involves transferring beneficial bacteria from the feces of healthy individuals into the patient’s gut, thereby regulating the micro-ecological balance of the gut and reconstructing gut microbiota to prevent and treat both intestinal and extraintestinal diseases. Currently, this therapy has been applied to the clinical treatment of over 80 diseases and has shown remarkable efficacy, particularly in patients who do not respond to traditional treatment options (such as recurrent Clostridium difficile infection). It is considered a safe and effective new technique.
The Tenth People’s Hospital of Shanghai has been exploring FMT treatments since 2012 and has treated over 20,000 patients. It has proposed a systematic response pattern for FMT therapy for the first time internationally: acute intestinal inflammation is more responsive than chronic intestinal inflammation; functional intestinal diseases respond better than structural intestinal diseases; intestinal diseases are more responsive than extraintestinal diseases; and extraintestinal diseases with intestinal symptoms respond better than those without. Specifically, FMT for treating recurrent Clostridium difficile infection can exceed a 90% success rate; for functional intestinal diseases such as constipation and irritable bowel syndrome, the efficacy can reach around 65%; for structural diseases like inflammatory bowel disease, the effectiveness is approximately 58.4%; for extraintestinal diseases like Parkinson’s diagnosed with intestinal symptoms, the efficacy is about 48.3%; and without intestinal symptoms, the efficacy in treating extraintestinal diseases is only 40.4%. A five-year follow-up of 8,547 patients confirmed that the adverse reactions to FMT are mostly short-term and self-limiting, with the primary symptoms being diarrhea, nausea, vomiting, and discomfort from nasogastric tube placement. Therefore, FMT is recognized as a safe and effective "green" treatment option, and its application is rapidly expanding globally, leading the transformation of healthcare concepts and diagnostic techniques in the 21st century.

Uncovering the Mysteries of Fecal Microbiota Transplantation Therapy
The FMT therapy consists of three components: donor, recipient, and treatment protocol, with the screening of effective donors being a key factor in determining the success or failure of FMT. Currently, the international donor screening for FMT has undergone three phases of conceptual innovation: in the initial phase, the screening criteria for FMT donors only included medical history and medication history; in the second phase, lifestyle history was also incorporated; and in the third phase, personal history and laboratory testing of blood/stool were added. As the origin of FMT, China follows a stricter and more standardized approach to donor screening, adhering to a "five steps, six dimensions" rule that comprehensively considers six health aspects: psychological, physiological, personal history, stability, sustainability, and food tolerability. According to current international standards, considering only factors such as age, body mass index (BMI), medical history, and medication history yields an effective rate of only 42.8% for FMT applications and a 30.7% rate of adverse events, including severe outcomes such as death. However, strictly adhering to the "five steps, six dimensions" screening rule can enhance the effectiveness rate to 68.7% and reduce the adverse event rate to 20.1%, with symptoms being mild to moderate and self-limiting. These healthy donors are crucial sources for correcting the patients’ dysbiotic microbiota.
Now, what kinds of diseases can benefit from FMT in restoring gut homeostasis and improving symptoms? Currently, clinical guidelines and expert consensus recommend FMT for the treatment of recurrent or refractory Clostridium difficile infections. Additionally, FMT shows good responsiveness in digestive system diseases (like inflammatory bowel disease, irritable bowel syndrome, functional constipation, liver cirrhosis), neuropsychiatric disorders (such as epilepsy, autism, depression, Parkinson’s disease, amyotrophic lateral sclerosis), metabolic diseases (like diabetes, obesity, fatty liver, hyperlipidemia), immune disorders, allergic diseases, and graft-versus-host disease. Furthermore, FMT can also serve as an adjuvant to enhance the effectiveness of tumor immunotherapy, improving patient response to immune checkpoint inhibitors.
However, it is essential to note that FMT is not suitable for all patients under certain contraindications; its blind use is highly discouraged in the following conditions: (1) patients with severe impairment of the gut barrier, such as those with sepsis, active gastrointestinal bleeding, or intestinal perforation; (2) patients with fulminant colitis or toxic megacolon; (3) patients who cannot withstand 50% of their caloric needs due to severe diarrhea, significant fibrous intestinal stenosis, acute gastrointestinal bleeding, or high-output enteric fistulas; (4) patients with congenital or acquired immune deficiencies; (5) those who have recently undergone high-risk immunosuppressive or cytotoxic drug treatment; (6) adults with neutrophils <1500/mm3 or children with neutrophils <1000/mm3; (7) pregnant or breastfeeding women.
After clarifying the characteristics of donors and recipients, how can we transfer the healthy donor's microbiota to patients? There are currently four primary transplant routes used internationally: nasogastric route, oral route, colonoscopy route, and enema route. For patients suffering from malnutrition or lesions that affect both the small and large intestines, the nasogastric route is preferred. For patients who cannot tolerate the nasogastric tube, such as elderly individuals and children, oral capsules are recommended for FMT. For those with lesions limited to the colon and with good FMT efficacy from a single session, colonoscopic FMT is recommended. Lastly, for lesions restricted to the rectum or sigmoid colon, enema FMT is suggested. In general, hospitalized patients are recommended to receive FMT via the nasogastric route, while patients undergoing consolidation therapy may use oral capsules for FMT.
Modern Manufacturing Processes Drive the Standardization of FMT Therapy Development
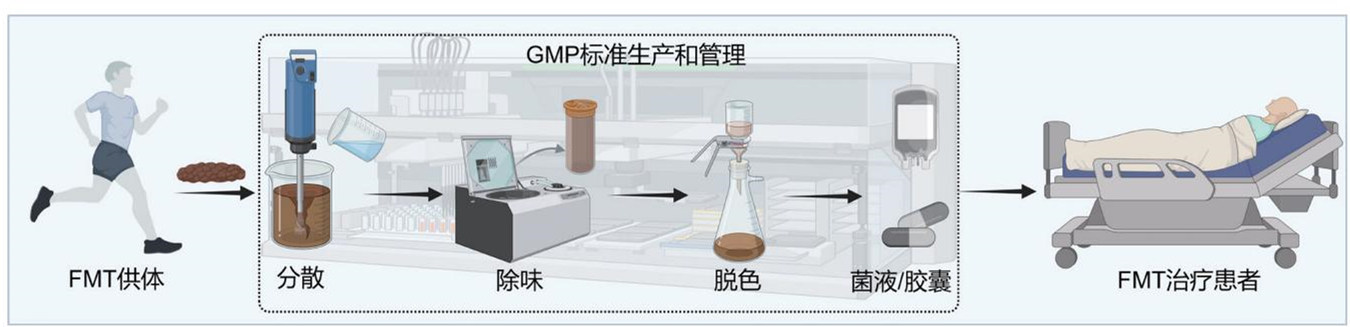
FMT is primarily achieved through liquid fecal microbiota or capsules containing freeze-dried preparations, where modern manufacturing processes can standardize and regulate the production of these transplants.
The healthy microbiota used for FMT is prepared from traceable fresh feces collected from donors using specially designed toilets to ensure the quantity, viability, and source of the microbiota while avoiding contamination by urine and sewage. The samples are then quickly cooled to 4°C and transported to a Good Manufacturing Practice (GMP) compliant production facility, where gut microbiota extraction is completed within six hours, strictly maintaining an anaerobic environment to ensure the viability of the microbiota during the process.
The extraction of healthy microbiota utilizes fully automated intelligent gut microbiota separation devices. At least 100 grams of feces mixed with physiological saline are introduced into this device for automatic stirring, filtering, and separation throughout the entire process. The processing is entirely conducted within a closed control unit to avoid exogenous bacterial contamination, leading to the generation of treatment-quality liquid microbiota. For each batch of liquid microbiota, 5% is randomly selected for preservation as a reference for post-treatment tracking.

The prepared liquid is centrifuged at 4°C and combined with skim milk powder and glycerol as a lyophilization protectant, utilizing freeze-drying technology to extract viable bacteria powder from the liquid, which is encapsulated in acid-resistant hydroxypropyl methylcellulose capsules to ensure that the viable microbiota can be released in the intestine, protecting them from gastric acid degradation.
Prior to FMT application, both the prepared liquid and capsules are randomly sampled for pathogenic bacteria testing, viable bacteria counting, and microbial genomic sequencing analysis to ensure the absence of pathogens like Clostridium difficile, Campylobacter, Salmonella, Shigella, Enterotoxigenic Escherichia coli, as well as eggs, cysts, parasites, spores, norovirus, rotavirus, and COVID-19, alongside negative results for multidrug-resistant genes. The bacterial activity of the transplant liquid should not be less than 80%, and the viable count should be no less than 5×10^8 per milliliter; the activity of the bacteria within capsules must be over 70%, with a viable count of no less than 1×10^9 per gram.
The standardized FMT transplant preparation process is documented in the "Clinical Application Management of Fecal Microbiota Transplantation - China Expert Consensus (2022 Edition)" and "Shanghai Technical Management Specifications for Microbiota Transplantation (2021 Edition)," facilitating the safe, effective, convenient, and controllable promotion of FMT technology within China.
Colonization Resistance: The 'Bottleneck' Issue in FMT Application
The gut microbiota is often referred to as the "hidden organ" of the human body, and thus the gut microbiota transplantation technique can be viewed as a type of "organ transplantation," inevitably accompanied by colonization resistance issues. Therefore, how to accurately transplant healthy microbiota to applicable patients is a significant technical challenge in the clinical application of FMT.
Microecological studies indicate that both the initial formation of gut microbial communities and the strength of recovery after interventions depend on the order of strain colonization within the gut. In simpler terms, the existing microbiota in the patient’s gut (referred to as indigenous microbes) can inhibit the healthy microbiota from the donor from colonizing in the recipient's gut through various means. Currently, the colonization rate of strains in FMT therapy ranges from 50% to 80%, and this efficacy declines significantly with increasing complexity of the disease and dysbiosis severity.
The primary mechanism by which indigenous microbes impact the colonization of donor microbes is through niche competition, which manifests as competition for nutrients and living space between the indigenous microbes and the healthy microbes from the donor. Before FMT therapy, the patient's indigenous microbes have already established a dominant position in the gut ecosystem, mainly occupying adhesion sites in the mucin layer and utilizing sialic acid modifications on these sites as a carbon source for growth. The newly transplanted healthy microbiota cannot quickly gain access to these sites, resulting in
