

Winners of the 2023 Nobel Prize in Chemistry: Professor Moungi G. Bawendi of MIT, Professor Louis E. Brus of Columbia University, and Nanocrystal Technology of the United States Alexei I. Ekimov, the company’s former chief scientist.
Quantum dots are like nano-prisons where electrons "dwell".
One 50,000th of the diameter of a human hair is about 1 nanometer, which is about 10 times the diameter of a hydrogen atom.
Quantum dots, which have been used in high-definition displays and computer screens to provide brilliant colors, are nanoscale materials, microcrystals and "artificial atoms."
Due to their unique optical and physical and chemical properties, quantum dots are also expected to be widely used in medical diagnosis, flexible electronic devices, solar cells, encrypted quantum communications and other fields.
Liang Wenjie, a researcher at the Institute of Physics, Chinese Academy of Sciences, is currently the leader of the N05 single-molecule and nanostructure electron transport research group in the Nanophysics and Devices Laboratory of the institute. On the evening of October 4, he told The Paper that so far, no natural quantum dots have been discovered.
In WeChat Moments, Liang Wenjie wrote, “If nature does not have materials that exhibit certain properties, humans can create them themselves by following the laws of nature. This idea is as important as inventing the wheel .” “From superlattice to quantum dots When it comes to corner materials, artificial materials based on the guiding ideas of artificial energy band and electronic correlation control are shining brightly."
Yuan Lanfeng is deputy director of the Science Communication Research Center of the Chinese Academy of Sciences, deputy director of the Department of Science and Technology Communication of the University of Science and Technology of China, associate researcher of the Hefei National Research Center for Microscale Physical Sciences of the University of Science and Technology of China, and president of the Society for Science, Technology and Strategy.
On the evening of the 4th, Yuan Lanfeng told The Paper that in addition to its application potential in real life, quantum dots are also extremely valuable at the theoretical cognitive level. The biggest revelation it brings is to reveal the "third dimension" of the periodic table of elements. The innovation it brings is the discovery of new ways to control material properties.
At about 17:45 on October 4, Beijing time, the Royal Swedish Academy of Sciences announced that the 2023 Nobel Prize in Chemistry will be awarded to Professor Moungi G. Bawendi of the Massachusetts Institute of Technology and Professor of Columbia University in the United States. Louis E. Brus and Alexei Ekimov, former chief scientist of Nanocrystal Technologies, Inc., for their discovery and synthesis of quantum dots contribute.
"Quantum dots can be seen as a milestone in the entire field of nanotechnology ." said Heiner Linke, professor of nanophysics, member of the Nobel Prize Committee for Chemistry, and member of the Royal Swedish Academy of Sciences.
A less "chemical" chemistry prize?
Regarding the 2023 Nobel Prize in Chemistry, some people believe that quantum dot research is not so "pure" chemical research, but closer to the realm of physics.
In this regard, Liang Wenjie said that this award is a long-awaited Nobel Prize in the field of nanoscience. "Actually, if it is awarded in the Nobel Prize in Physics, there is no problem at all. Because this is a huge breakthrough for humans to control the properties of materials and an important milestone in the field of artificial materials. However, there is no problem in awarding it in the Chemistry Prize. , because it provides a chemical technology to achieve large-scale synthesis of quantum dots. It is indeed not a deeper understanding of chemical reactions or chemical kinetics, but a chemical approach that lays the foundation for the development of nanotechnology and has achieved great results. breakthrough."
Liang Wenjie told The Paper that there was a theoretical breakthrough and he discovered a quantum confinement effect and explained it; and large-scale preparation technology made this quantum confinement effect accessible to people. He believed that these were two of the achievements of quantum dots. A highlight. The greater significance is to stimulate people's imagination and bring new dimensions to material performance design.
Quantum dots (schematic, left) are nanoscale microcrystals, typically composed of thousands of atoms.
Nanoscale "carving" and "dwelling" electrons
Quantum dots are usually nanocrystals composed of thousands of atoms.
Liang Wenjie explained, "The 'quantum' in 'quantum dot' refers to the quantum effect. 'Dot' means that matter, electrons or atoms are confined in a very small space, and the size is so small that it is almost negligible. With quantum Changes in the size or shape of a dot can significantly change its physical and chemical properties."
"It's like a group of us holding a banquet. Originally in a relatively empty and large room, everyone was free and peaceful. But then the space became smaller and smaller, and people gradually crowded and influenced each other. Everyone's Faces change, moods get irritable. Size determines the properties of quantum dots. Because electrons are like us at a party."
Quantum dots absorb light and then radiate light at another wavelength. Even if they are of the same composition, quantum dots of different sizes will still show different colors. Although their chemical composition and elemental composition have not changed, the size changes at the nanoscale have changed their electronic configuration.
The predictions made by theoretical calculations preceded the actual experimental discovery and successful synthesis of quantum dots by about 40 years.
In 1937, physicist Herbert Fröhlich calculated that when the size of material particles becomes extremely small, electrons, which are both waves and particles, will be squeezed together, which will lead to The properties of the material change dramatically.
Fascinated by his insights, other researchers used mathematical tools to predict many size-related quantum effects and diligently tried to demonstrate them experimentally. But 1 nanometer is equal to one millionth of a millimeter, which is equal to one billionth of a meter. They needed to carve a tiny structure a million times smaller than the tip of a needle to conduct the experiment. This was a huge technical problem at the time.
It was not until the early 1980s that Russian and American scientists independently created the first quantum dots. Alexei Ikimov, who had just graduated from his PhD and was working at the Vavilov State Institute of Optics in the Soviet Union, observed that the size of copper chloride microcrystals in glass samples prepared by different firing processes varied greatly. Some are only about 2 nanometers, and some are as high as 30 nanometers.
He found that the smaller the microcrystalline particles, the bluer the light they absorbed.
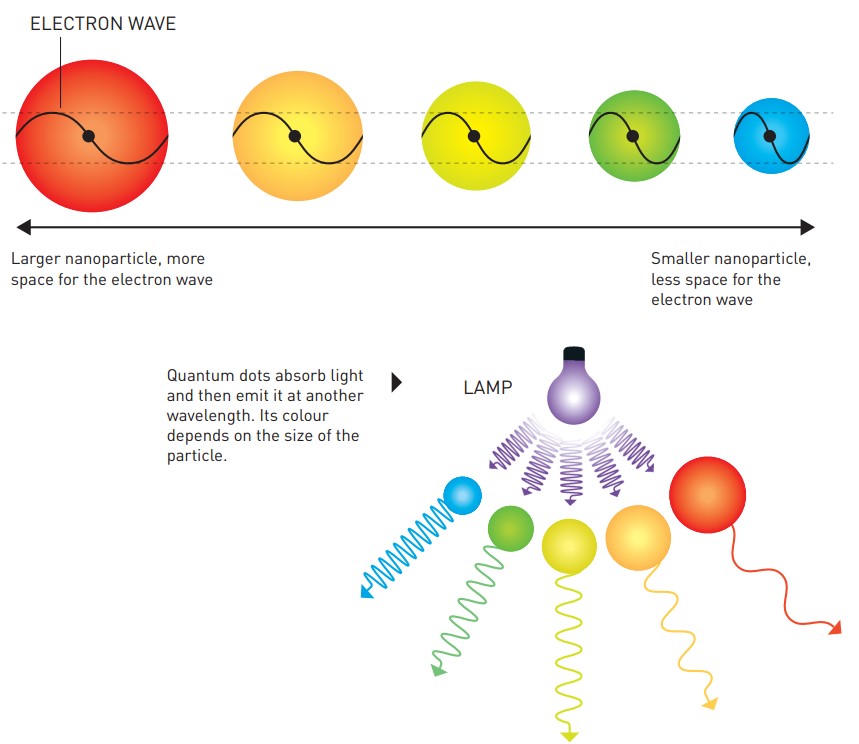
Quantum dots absorb light and then radiate light at another wavelength. The larger the particle size, the more space is left for electron waves. Photo: Johan Jarnestad
In 1981, Ikimov published the above discovery in a Soviet scientific magazine and explained it as a quantum effect related to the size of nanomaterials - the quantum size effect.
Two years later, in 1983, Louis E. Bruce published similar experimental results on quantum effects. He compared 4.5-nanometer and 12.5-nanometer cadmium sulfide particles, demonstrating for the first time the quantum size effect of freely floating colloidal particles in liquids.
Why is it so important and of interest that the absorbance of material particles is slightly skewed towards blue?
Because electrons govern the optical properties of a substance, they also govern its ability to catalyze reactions or conduct electricity. When researchers detect changes in a substance's absorbance, they understand that, in principle, they are looking at an entirely new material.
Biggest contribution: The “third dimension” of the periodic table of elements
In addition to the colorful optical properties and applications, Yuan Lanfeng believes that the biggest contribution of quantum dots is "equivalent to 'adding a dimension to the periodic table of elements'. I think this is the best expression." This is the theoretical cognitive level enlightenment it brings.
The properties of a chemical element are mainly affected by the number of its electron shells and the number of electrons in its outer shell. These are considered the two dimensions of the periodic table of elements. Previously, people's expansion of the periodic table of elements was limited to the two-dimensional level, and they were constantly looking for or creating new elements.
But quantum dot materials show that at the nanometer level, changes in size have a significant impact on the properties of the material, meaning scientists who want to develop new materials have one more factor to take advantage of.
Yuan Lanfeng said, "Everyone compares materials (design) to ' stir-frying '. In the past, many elements were used to 'stir-fry'. Now, another dimension has been added - the size of nanoparticles can be adjusted. Then the dishes can be stir-fried. Isn’t it more?”
Liang Wenjie said that if the size of the quantum dot changes a little, the "temper" of the electron may change a lot. "How we can control it more precisely to make its performance more and more excellent, and then produce disruptive technologies, is a question that needs further exploration."
Yuan Lanfeng told The Paper, “One thing that impressed me deeply was my conversation with Nobel Prize winner Andre Geim in 2020. At the beginning, I prepared a question and wanted to ask him about the industrialization of graphene. question, or to what extent has the technical application of graphene been achieved? Because many people said that graphene material lacks a killer application. Unexpectedly, he was not interested in this kind of problem at all. He said, 'I won the Nobel The award is not for the application of technology, but for me revealing a possibility. Previously, people thought that materials were all three-dimensional, but I tell everyone that there are new fields and two-dimensional materials. You will find that two-dimensional materials are far away It’s not just graphene. You don’t have to be limited to graphene at all, you can look for more two-dimensional materials and find other more useful things.’ So Andre Geim’s biggest contribution is to tell everyone about the existence of two-dimensional materials. Likewise. , the biggest contribution of quantum dots is to tell everyone that the periodic table of elements has a third dimension. "
For their pioneering experiments on two-dimensional graphene materials, Andre Geim, a professor at the University of Manchester in the United Kingdom, and his student Konstantin Novoselov were awarded the 2010 Nobel Prize in Physics.
In addition, Yuan Lanfeng also mentioned another new concept caused by quantum size effect: nano-confined catalysis. At the 2020 National Science and Technology Awards Conference, the relevant project team of the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, led by Academician Bao Xinhe, President of the University of Science and Technology of China, won the first prize of the Natural Science Award. According to CCTV news reports, nano-confined catalysis actually provides a constrained environment for the catalytic reaction system at the nanoscale, such as the role of space and interfaces, etc., to modulate the electronic energy state of the catalyst system and change the activity and activity of the catalyst. Selectivity, thereby achieving precise control of catalytic performance.
Does the method of synthesis matter?
What is engineering? Liang Wenjie said that first of all, it needs to be prepared in large quantities and consistent.
Yuan Lanfeng explained that there is a very important indicator for making chips now - chip yield. If a method is only theoretically feasible but has a particularly low yield, it may be extremely costly and either completely unusable or become a game for a few people. Just like before the invention of large-scale electrolysis of aluminum, only nobles and royal families could afford aluminum products. For quantum dots to " fly into ordinary people's homes ", the yield rate must be improved.
How to synthesize large batches of nanocrystals of specific sizes?
In the 1980s, the "knife skills" of nanocarving were not good enough, thus hindering quantum dot-related research and development work.
The breakthrough didn't come until ten years later.
In 1983, Bruce published a research paper on the quantum size effect. Five years later, in 1988, Mongi G. Bawendi came to Bruce's laboratory to start postdoctoral research. The quality of the nanocrystals they produced was getting better, but still not ideal.
Another five years later, in 1993, the research team led by Bawendi finally made a major breakthrough. They used a hot injection synthesis method to successfully synthesize monodispersed nanoparticles, opening the door to the development of large-scale applications of quantum dots.
Liang Wenjie introduced that the hot injection synthesis method is to heat a specific solvent to above 300 degrees Celsius, and then inject a solution containing quantum dot materials into the aforementioned boiling solvent. Due to supersaturation, quantum dot materials quickly form nuclei, like condensation nuclei in rain clouds.
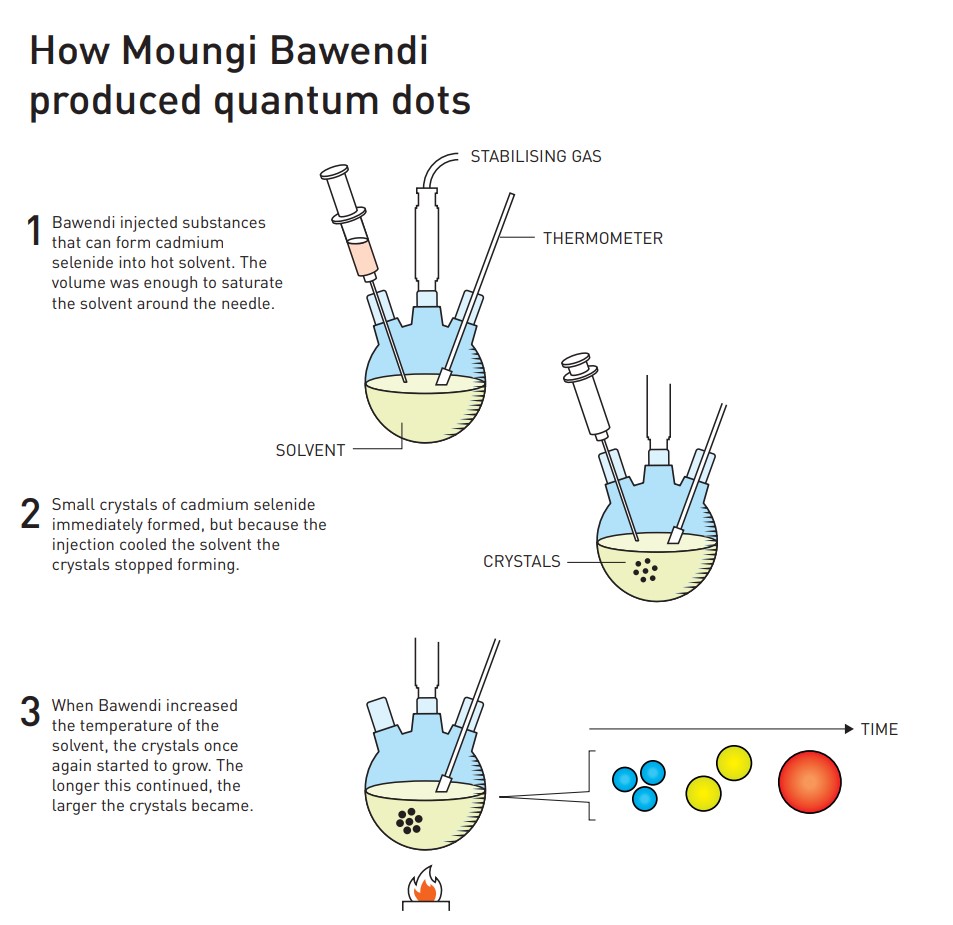
Quantum dots were synthesized by hot injection synthesis. Photo: Johan Jarnestad
"But we don't want the crystal to grow all the time, otherwise it won't be a nanoparticle. So the researchers added some more 'blockers' to the solution. The longer the crystal grows, the stronger the blocking effect becomes, and finally it stabilizes in a chemical equilibrium state. Produce nanoparticles with good consistency in the solution. By adjusting the reaction conditions, whether adding surfactant or controlling the temperature, the size of the nanoparticles can be adjusted, such as 3 nanometers, 5 nanometers, and 10 nanometers." Liang Wenjie said.
Heiner Link wrote in an article published on the official website of the Nobel Prize that the field of modern nanoscience requires precise and ideal atomic-level modulation of the synthesis of nanostructures. The discovery of quantum dots, and the ability to synthesize this material with highly precise but relatively simple chemical methods, is an important step in the development of nanoscience and nanotechnology. The winner of the 2023 Nobel Prize in Chemistry has played a central role in establishing these capabilities and, in this way, provided the seeds for the growth of the field of nanoscience.