As hundreds of millions of people around the world are infected with the new coronavirus, the problem of "Long Covid" (long-term impact of the new crown) is beginning to surface.
The latest data from the US Centers for Disease Control and Prevention shows that at least one in five people infected with the new crown in the United States has long-term symptoms of the new crown. In the United Kingdom, 2 million people have symptoms of new crowns, and 619,000 people reported long new crowns during the Omicron epidemic, accounting for 31%.
Brain fog (a phenomenon in which the brain has difficulty forming clear thinking and memory), fatigue, difficulty concentrating, and loss of taste and smell are common symptoms of COVID-19. The mutant strain of the new coronavirus that swept across Taiwan recently caused at least 17 children to develop symptoms of encephalitis. There are various indications that the new coronavirus infection has a profound impact on the human nervous system. After the acute infection period of the new crown, why is there a "long new crown"? And how many neurological symptoms? What is the mechanism behind this? These are the core frontiers of current global academic attention.
Recently, the latest issue of the international authoritative academic journal "Science Translational Medicine" (impact factor 17.956) published a major research focusing on the biological mechanism of "long new crown" caused by new coronavirus infection. The research comes from top scientific research teams in the industry such as New York University, Columbia University, Harvard University, and the Icahn School of Medicine at Mount Sinai in New York.
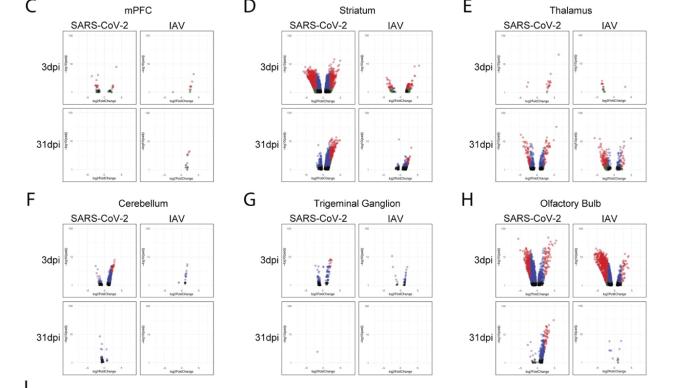
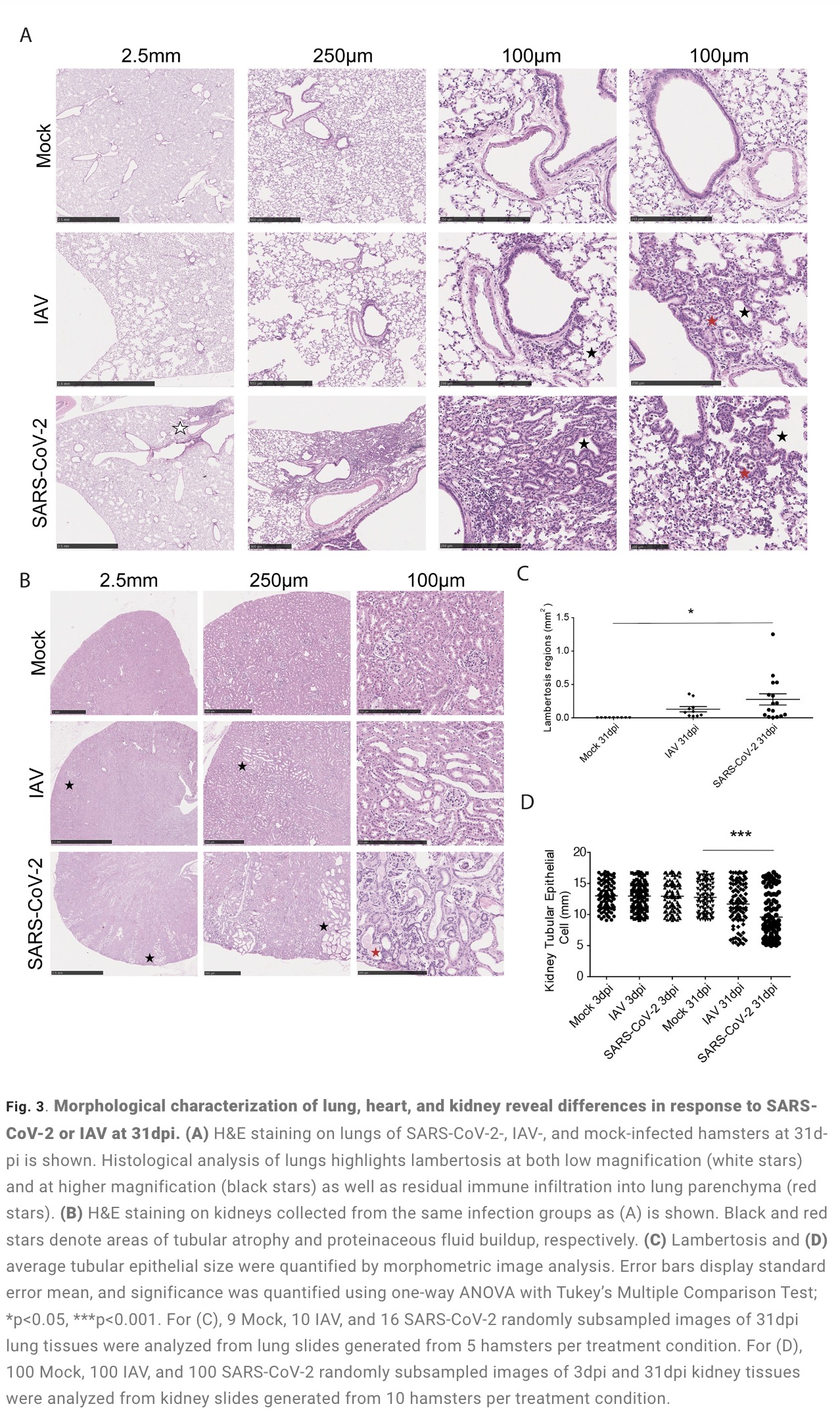
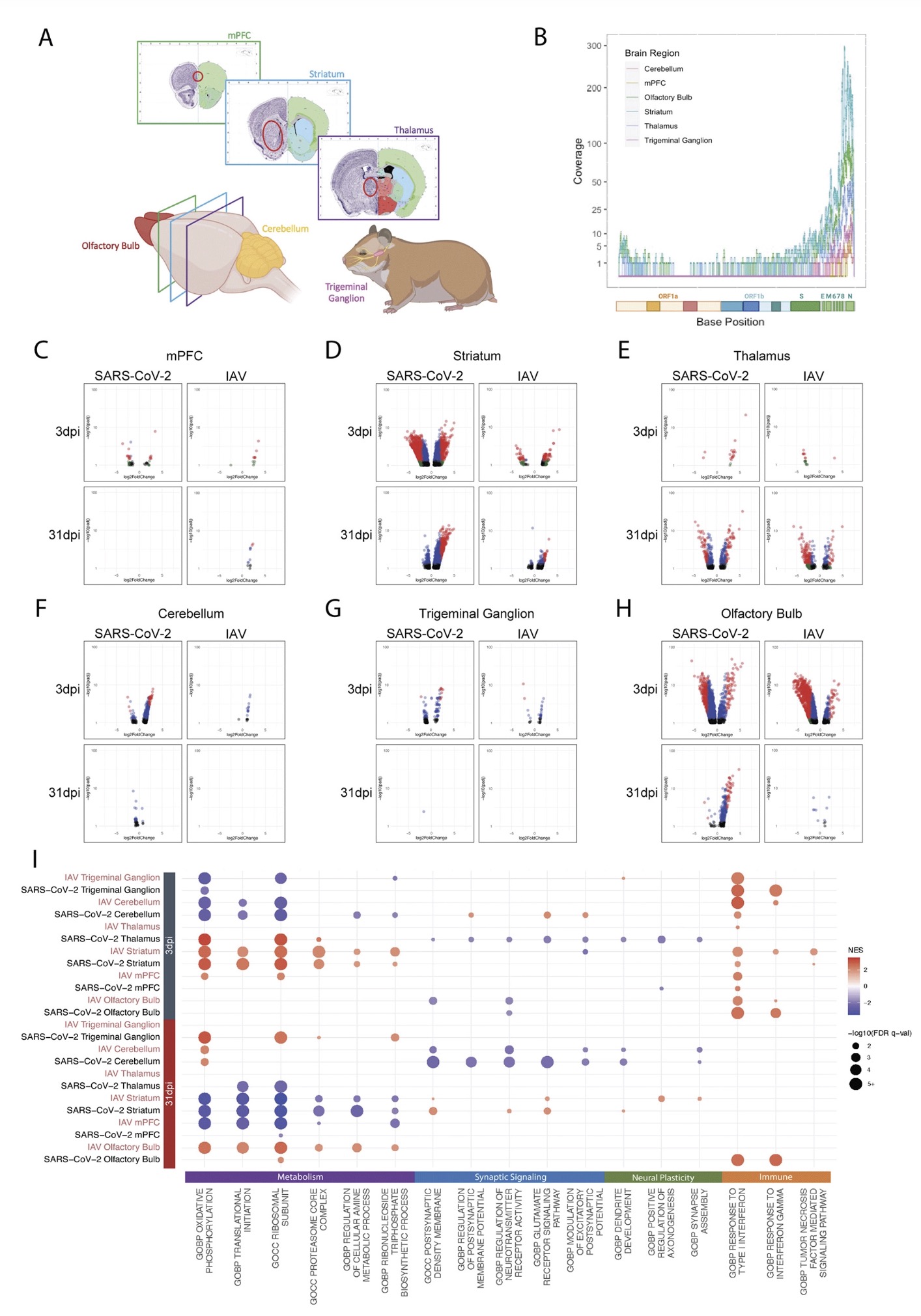
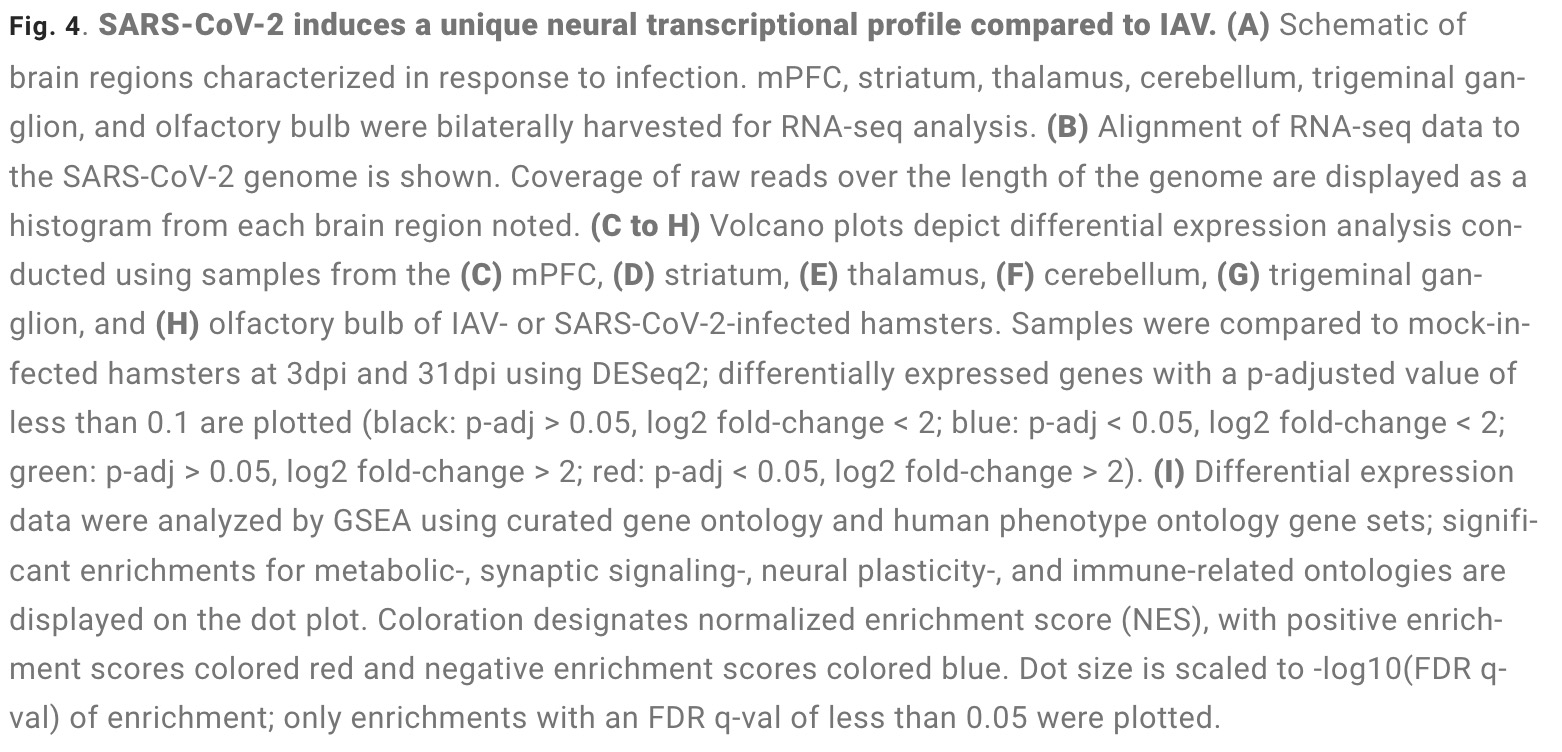
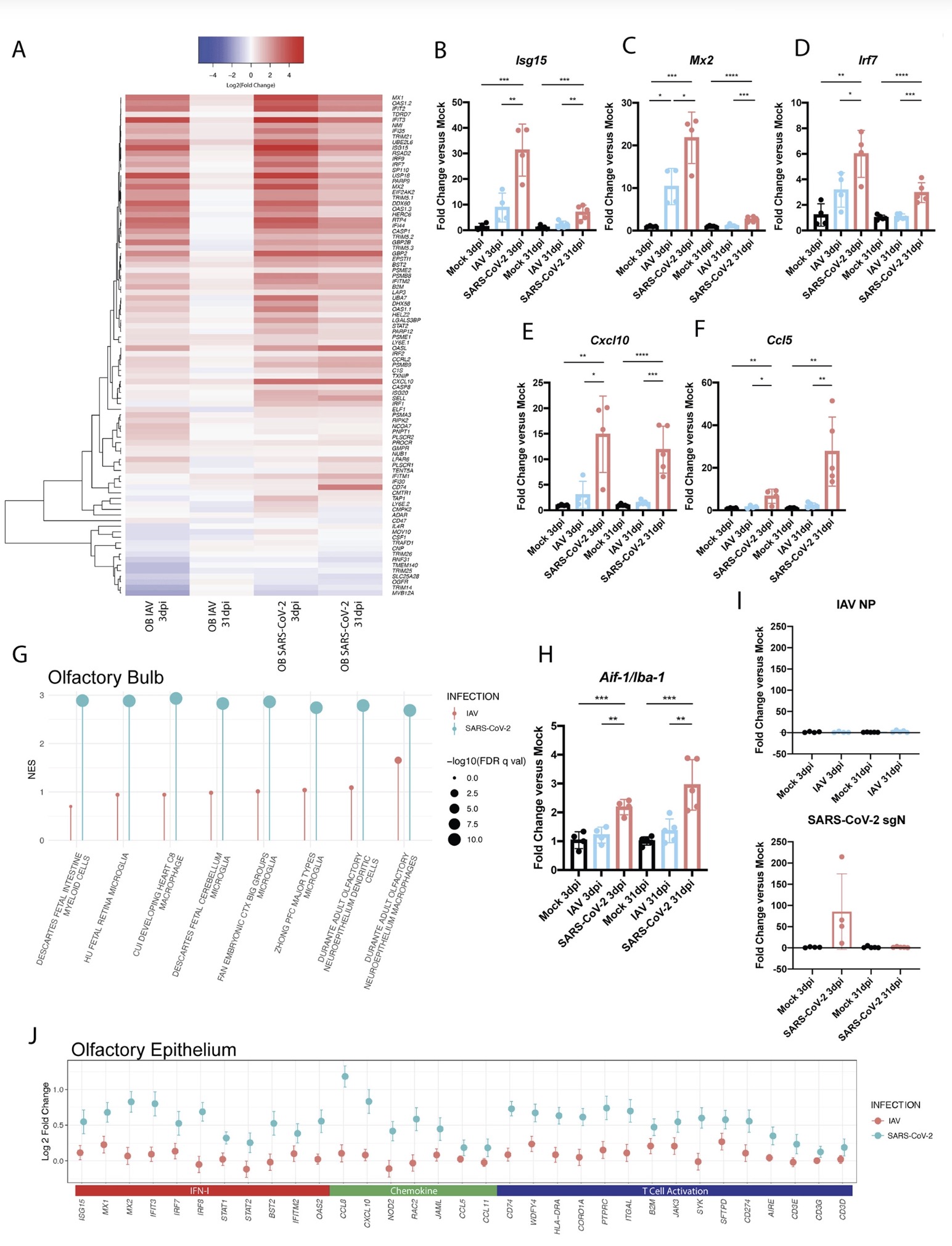
The study found that: Compared with influenza A virus, the new coronavirus can cause more severe systemic acute infection, and its ability to cause permanent damage to the lungs and kidneys exceeds that of influenza A virus (IAV), and the new coronavirus can also induce a unique Neural Transcription. It has unique effects on tissues involved in the sense of smell, such as the olfactory bulb (OB) nervous system and olfactory epithelium (OE) neurons in the brain. Although no infectious virus was detected in these neural tissues involved in olfaction, pathological dissection of the olfactory bulb and olfactory epithelium revealed the presence of inflammatory markers such as myeloid and T cell activation, and proinflammatory cytokines.
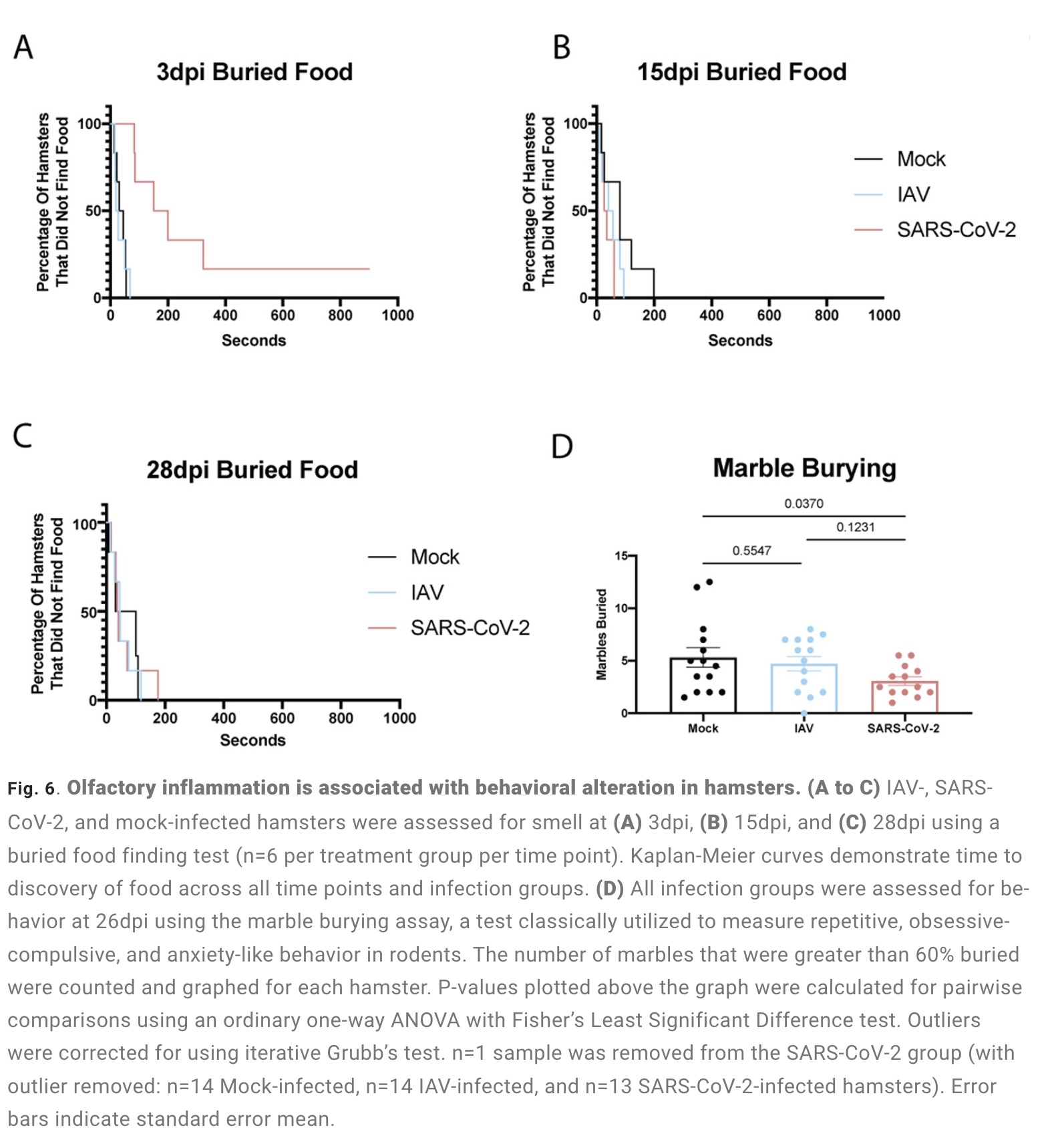
The olfactory bulb is a part of the telencephalic cortex, located below and anterior to the olfactory groove in the orbital surface of the frontal lobe of the cerebral hemisphere. The olfactory epithelium is located in the nasal cavity and contains olfactory receptor cells with specialized ciliary extensions. Cilia capture odor molecules as they cross the epithelial surface. Information about the molecule is then passed from the receptor to the olfactory bulb in the brain. Chronic inflammation within the olfactory bulb affects sensory, emotional, and cognitive processes. The study found through animal model experiments that there was persistent inflammation in the olfactory tissue of new coronavirus-infected hamsters, and in the presence of these inflammations, the behavior of the hamsters measured by marble burial also changed. The marble burial assay is a classic test used to measure repetitive, obsessive-compulsive, and anxiety-like behaviors in rodents. Previous academic studies have shown that burying behavior is the behavior of rodents in the face of novel and special objects, especially when faced with unfavorable stimuli, and the conditioned burying behavior of hamsters reflects an anxiety-related emotion.
The study found through animal model experiments that there was persistent inflammation in the olfactory tissue of new coronavirus-infected hamsters, and in the presence of these inflammations, the behavior of the hamsters measured by marble burial also changed. The marble burial assay is a classic test used to measure repetitive, obsessive-compulsive, and anxiety-like behaviors in rodents. Previous academic studies have shown that burying behavior is the behavior of rodents in the face of novel and special objects, especially when faced with unfavorable stimuli, and the conditioned burying behavior of hamsters reflects an anxiety-related emotion.
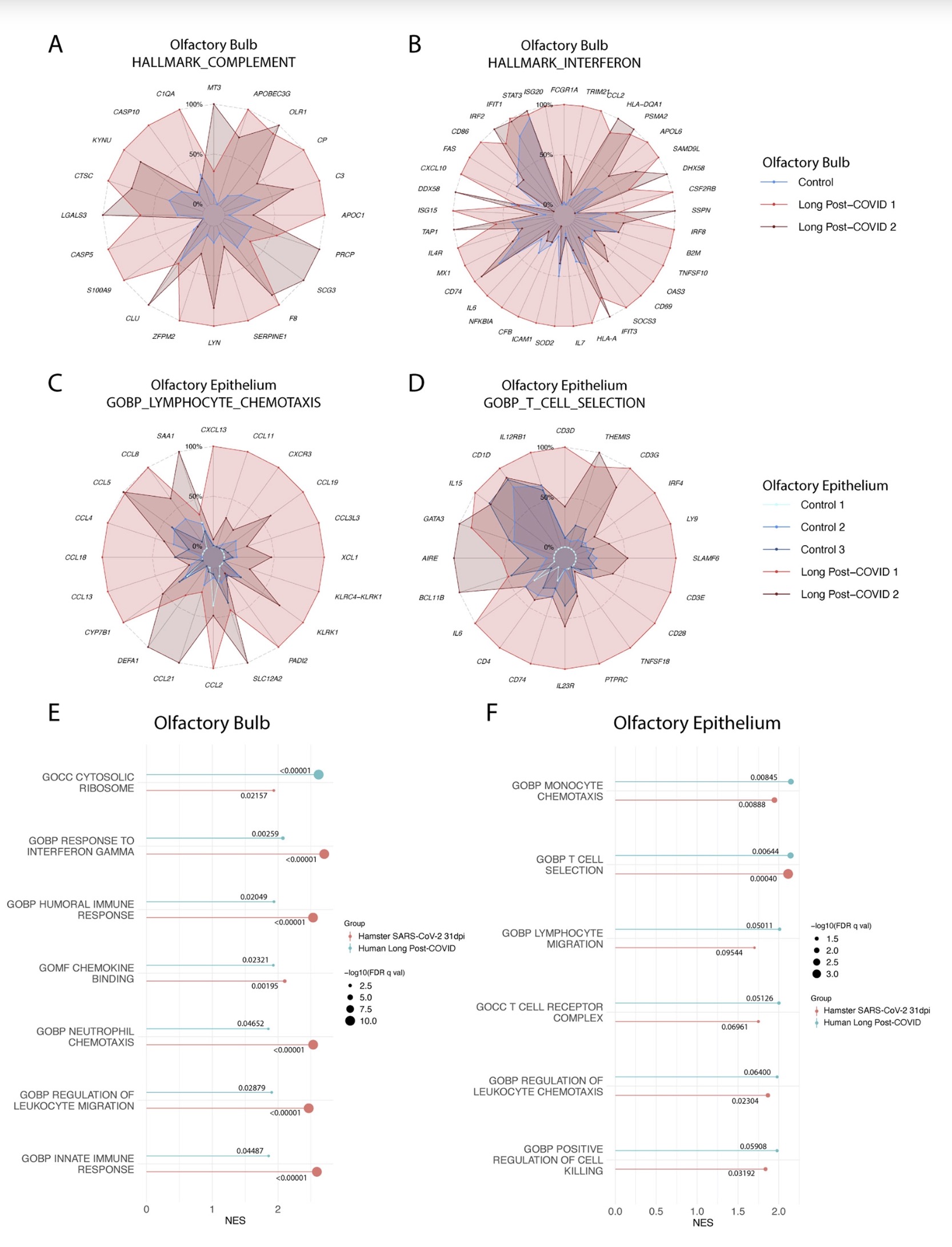
In fact, in both humans and hamsters, the olfactory bulb is functionally connected to the limbic system that controls appetite, sensory, emotional, and cognitive responses, and can therefore influence neurolimbic activity. These findings suggest that behavioral changes after infection may be causally linked to olfactory inflammation. Previous preclinical studies have linked olfactory bulb damage to depressive phenotypes that can be reversed by antidepressant treatment. The researchers suggest that chronic olfactory epithelial nerve and olfactory bulb inflammation may drive neurodegeneration and structural changes consistent with COVID-19 symptoms. Recent clinical evidence further confirms that patients recovering from mild COVID-19 show gray matter loss in limbic cortical regions associated with olfactory system function.
Findings in peripheral organs and the central nervous system have identified transcriptional and histological signatures caused by SARS-CoV-2 infection that may induce various somatosensory, affective, and cognitive impairments that persist long after the original infection. Given the systematic scope of these findings, the study elucidates the molecular basis that underlies many of the heterogeneous symptoms of COVID-19.
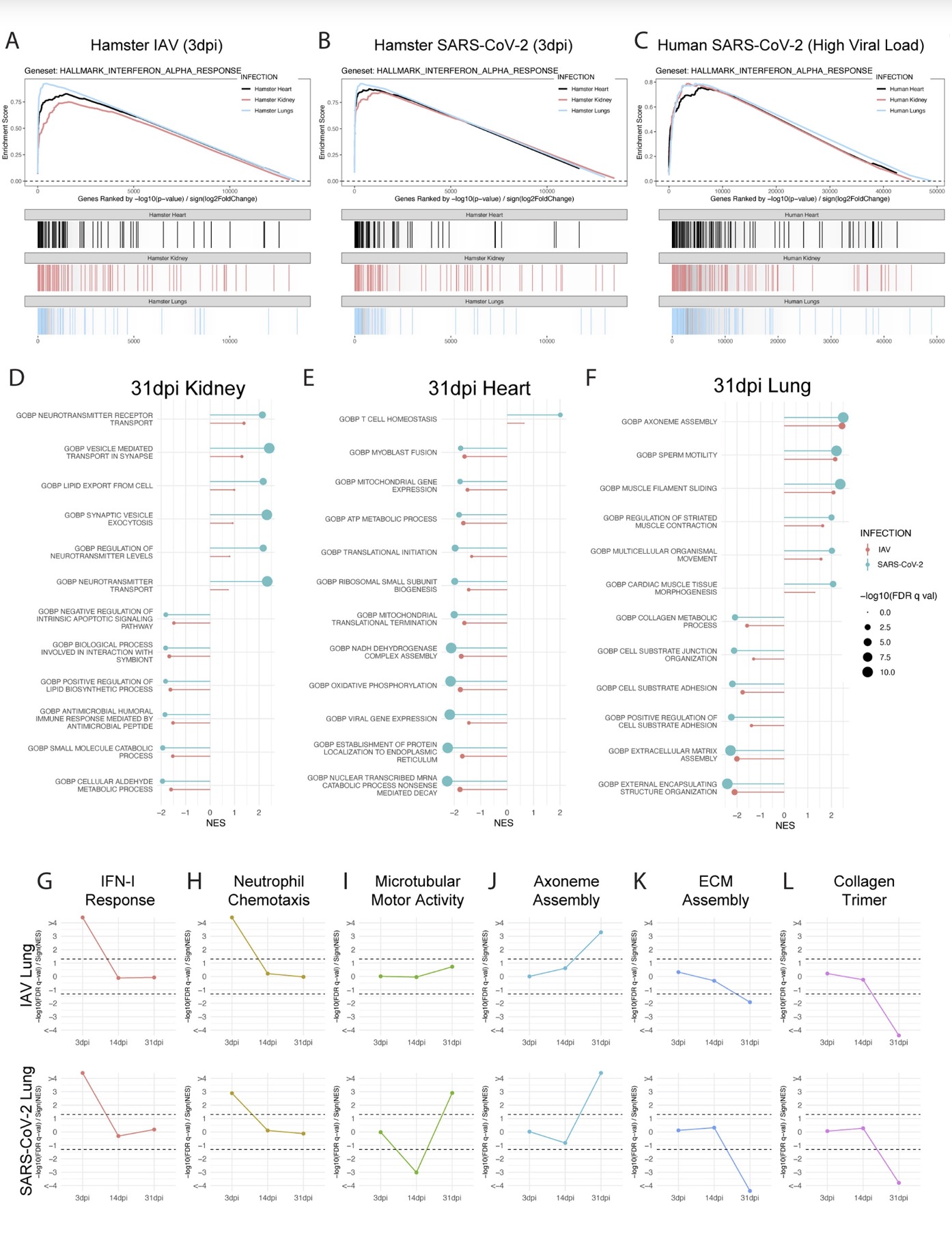
The study found that the new coronavirus can also affect the striatum, one of the basal ganglia of the brain. The main function of the striatum is to regulate muscle tone and coordinate various fine and complex movements. If the striatum is damaged, its function is impaired. SARS-CoV-2 induces changes in the striatum of the brain associated with metabolic and functional shifts.
SARS-CoV-2 infection also has an inhibitory effect on the thalamus, which may lead to cognitive impairment, possibly in the form of functional changes within the thalamus or functional connections to key brain regions that drive emotion, motivation, cognition, sleep, pain, arousal, and motor activity appear in the form. Regional transcriptional changes in thalamic nuclei may contribute to the development of neuropsychiatric disorders in recovered patients from 2019-nCoV infection. These findings provide clues for understanding the molecular mechanism of "long new crown".
Attachment: Important charts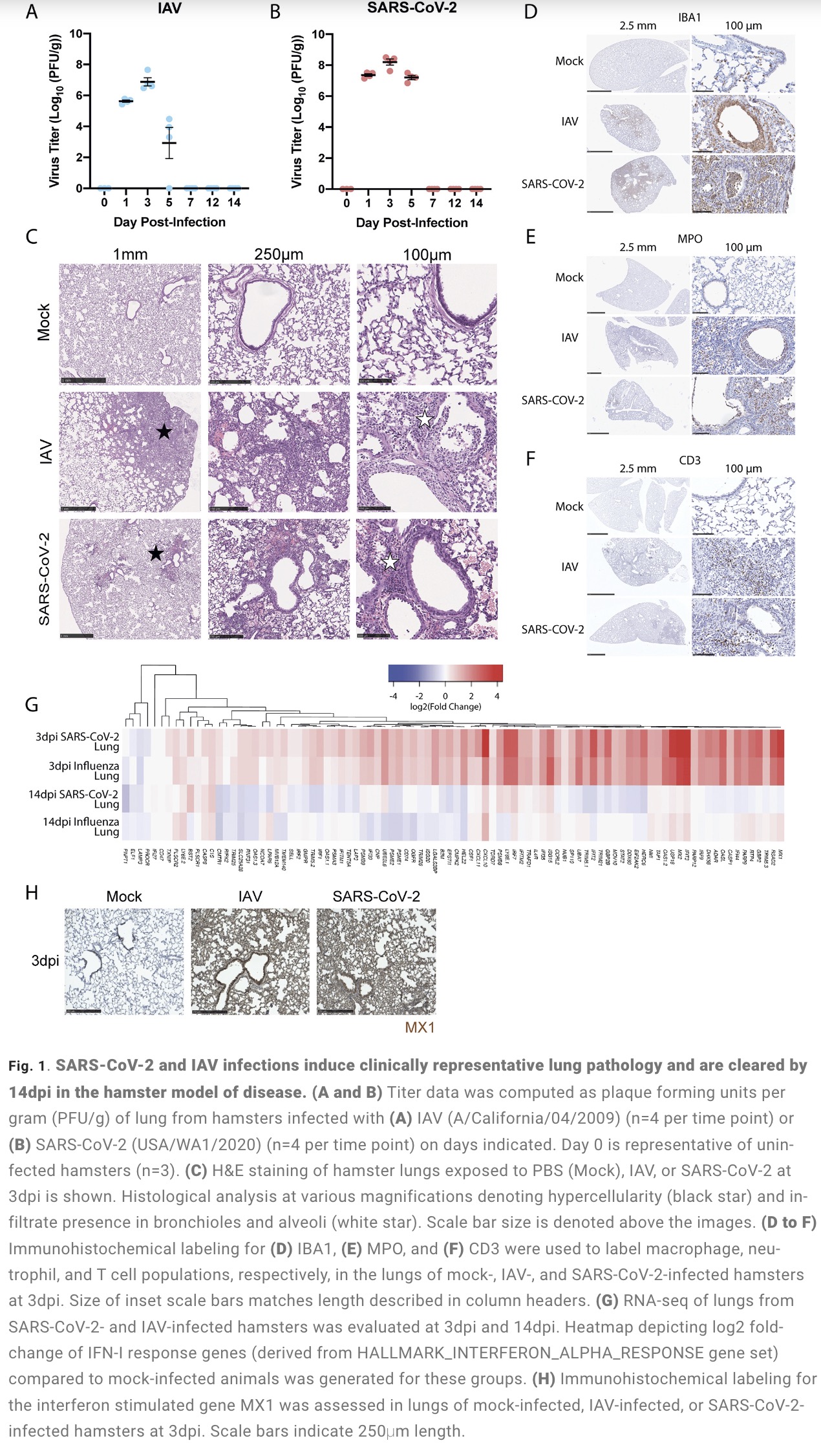

The latest data from the US Centers for Disease Control and Prevention shows that at least one in five people infected with the new crown in the United States has long-term symptoms of the new crown. In the United Kingdom, 2 million people have symptoms of new crowns, and 619,000 people reported long new crowns during the Omicron epidemic, accounting for 31%.
Brain fog (a phenomenon in which the brain has difficulty forming clear thinking and memory), fatigue, difficulty concentrating, and loss of taste and smell are common symptoms of COVID-19. The mutant strain of the new coronavirus that swept across Taiwan recently caused at least 17 children to develop symptoms of encephalitis. There are various indications that the new coronavirus infection has a profound impact on the human nervous system. After the acute infection period of the new crown, why is there a "long new crown"? And how many neurological symptoms? What is the mechanism behind this? These are the core frontiers of current global academic attention.
Recently, the latest issue of the international authoritative academic journal "Science Translational Medicine" (impact factor 17.956) published a major research focusing on the biological mechanism of "long new crown" caused by new coronavirus infection. The research comes from top scientific research teams in the industry such as New York University, Columbia University, Harvard University, and the Icahn School of Medicine at Mount Sinai in New York.
The study found that: Compared with influenza A virus, the new coronavirus can cause more severe systemic acute infection, and its ability to cause permanent damage to the lungs and kidneys exceeds that of influenza A virus (IAV), and the new coronavirus can also induce a unique Neural Transcription. It has unique effects on tissues involved in the sense of smell, such as the olfactory bulb (OB) nervous system and olfactory epithelium (OE) neurons in the brain. Although no infectious virus was detected in these neural tissues involved in olfaction, pathological dissection of the olfactory bulb and olfactory epithelium revealed the presence of inflammatory markers such as myeloid and T cell activation, and proinflammatory cytokines.
The olfactory bulb is a part of the telencephalic cortex, located below and anterior to the olfactory groove in the orbital surface of the frontal lobe of the cerebral hemisphere. The olfactory epithelium is located in the nasal cavity and contains olfactory receptor cells with specialized ciliary extensions. Cilia capture odor molecules as they cross the epithelial surface. Information about the molecule is then passed from the receptor to the olfactory bulb in the brain. Chronic inflammation within the olfactory bulb affects sensory, emotional, and cognitive processes.
 The study found through animal model experiments that there was persistent inflammation in the olfactory tissue of new coronavirus-infected hamsters, and in the presence of these inflammations, the behavior of the hamsters measured by marble burial also changed. The marble burial assay is a classic test used to measure repetitive, obsessive-compulsive, and anxiety-like behaviors in rodents. Previous academic studies have shown that burying behavior is the behavior of rodents in the face of novel and special objects, especially when faced with unfavorable stimuli, and the conditioned burying behavior of hamsters reflects an anxiety-related emotion.
The study found through animal model experiments that there was persistent inflammation in the olfactory tissue of new coronavirus-infected hamsters, and in the presence of these inflammations, the behavior of the hamsters measured by marble burial also changed. The marble burial assay is a classic test used to measure repetitive, obsessive-compulsive, and anxiety-like behaviors in rodents. Previous academic studies have shown that burying behavior is the behavior of rodents in the face of novel and special objects, especially when faced with unfavorable stimuli, and the conditioned burying behavior of hamsters reflects an anxiety-related emotion.In fact, in both humans and hamsters, the olfactory bulb is functionally connected to the limbic system that controls appetite, sensory, emotional, and cognitive responses, and can therefore influence neurolimbic activity. These findings suggest that behavioral changes after infection may be causally linked to olfactory inflammation. Previous preclinical studies have linked olfactory bulb damage to depressive phenotypes that can be reversed by antidepressant treatment. The researchers suggest that chronic olfactory epithelial nerve and olfactory bulb inflammation may drive neurodegeneration and structural changes consistent with COVID-19 symptoms. Recent clinical evidence further confirms that patients recovering from mild COVID-19 show gray matter loss in limbic cortical regions associated with olfactory system function.
Findings in peripheral organs and the central nervous system have identified transcriptional and histological signatures caused by SARS-CoV-2 infection that may induce various somatosensory, affective, and cognitive impairments that persist long after the original infection. Given the systematic scope of these findings, the study elucidates the molecular basis that underlies many of the heterogeneous symptoms of COVID-19.
The study found that the new coronavirus can also affect the striatum, one of the basal ganglia of the brain. The main function of the striatum is to regulate muscle tone and coordinate various fine and complex movements. If the striatum is damaged, its function is impaired. SARS-CoV-2 induces changes in the striatum of the brain associated with metabolic and functional shifts.
SARS-CoV-2 infection also has an inhibitory effect on the thalamus, which may lead to cognitive impairment, possibly in the form of functional changes within the thalamus or functional connections to key brain regions that drive emotion, motivation, cognition, sleep, pain, arousal, and motor activity appear in the form. Regional transcriptional changes in thalamic nuclei may contribute to the development of neuropsychiatric disorders in recovered patients from 2019-nCoV infection. These findings provide clues for understanding the molecular mechanism of "long new crown".
Attachment: Important charts










Related Posts
0 Comments
Write A Comments