The New England Journal of Medicine, an international authoritative medical journal, published a communication on June 9, local time: A Japanese research institution detected a case of prion in the brain of 75 cadavers received for autopsy.
Researchers from the Institute of Biomedical Sciences at Nagasaki University in Japan reported that when they performed prion RT-QuIC testing on 75 cadavers received by an institution for dissection practice, they found that one of the cerebral cortex slices actually contained Prion.
The essence of prions is proteins. If the proteins in the central nervous system of humans and some animals are misfolded, they will form infectious prions. They continuously combine with adjacent normal proteins in the brain, making the brain appear spongy. Hollow, causing brain function degradation, dementia and other symptoms, and eventually death.
The donor with prions in his brain died eight years ago at the age of 92. He died of aspiration pneumonia and no surviving relatives provided medical or family history information.
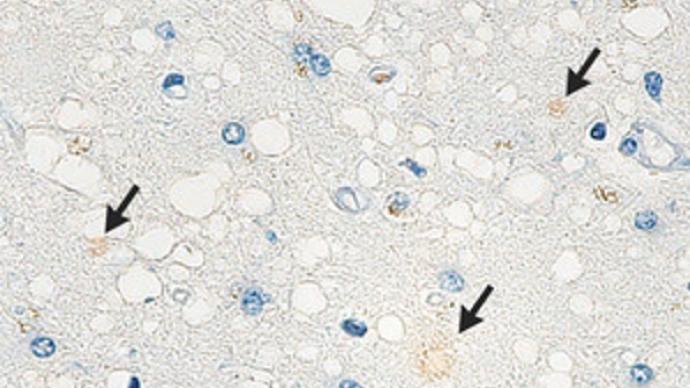
The researchers observed the old man's hematoxylin and eosin-stained specimens under a microscope and found extensive spongy changes in the cerebral cortex, vacuoles of different sizes did not tend to merge, and neurons were mostly preserved, while some mild Degree of gliosis. Without frozen tissue or blood samples, researchers would not be able to confirm the presence of mutations in the genes encoding prion proteins.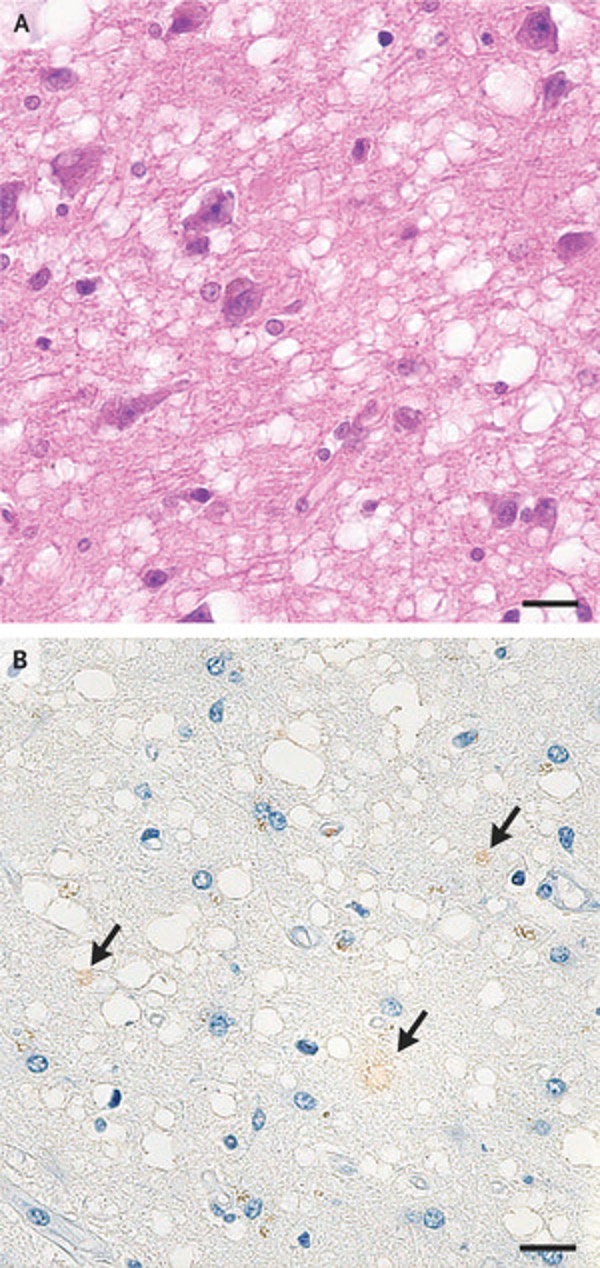 Using immunohistochemical staining of the processed specimens, the researchers found that the old man's prion may carry the V180I mutation. Abnormal prion protein (PrPSc) showed only weak signal in the temporal cortex. Because of the small sample size of prions, the researchers could not determine whether the prions in their bodies were contagious.
Using immunohistochemical staining of the processed specimens, the researchers found that the old man's prion may carry the V180I mutation. Abnormal prion protein (PrPSc) showed only weak signal in the temporal cortex. Because of the small sample size of prions, the researchers could not determine whether the prions in their bodies were contagious.
"It is concerning that there is a possibility of exposure to prions through accidental contact with prion-infected tissue, which puts others at risk of transmission," the researchers said in the communication.
The discovery of prions in cadavers means that all personnel involved in autopsy processing and autopsies need to wear personal protective equipment and follow safety protocols. They need to avoid direct body contact with dead bodies and bodily fluids, as well as avoid injuries such as cuts when examining tissue and taking samples.
Prions are resistant to all standard sterilization measures, and other patients, surgeons, pathologists, and technicians handling infected tissues and instruments are at risk of infection.
To ensure safety, sterilize them by autoclaving at 132°C for 1 hour, or soak them in sodium hydroxide 1 N (normal) or 10% sodium hypochlorite solution for 1 hour.
The global incidence of known prion diseases is estimated at one to two cases per million people per year, the researchers wrote in the newsletter. The incidence of undiagnosed prion diseases, although unknown, is estimated to be as high as one case per 30,000 people.
In this case, it is important to determine whether the etiology is iatrogenic, familial, or sporadic.
Prion diseases are present in many mammals (eg, mink, elk, deer, domesticated cattle and sheep) and can be transmitted between different species through the food chain. There have been previous cases of people at risk of becoming infected after eating beef with bovine spongiform encephalopathy (BSE, or mad cow disease).
Researchers from the Institute of Biomedical Sciences at Nagasaki University in Japan reported that when they performed prion RT-QuIC testing on 75 cadavers received by an institution for dissection practice, they found that one of the cerebral cortex slices actually contained Prion.
The essence of prions is proteins. If the proteins in the central nervous system of humans and some animals are misfolded, they will form infectious prions. They continuously combine with adjacent normal proteins in the brain, making the brain appear spongy. Hollow, causing brain function degradation, dementia and other symptoms, and eventually death.
The donor with prions in his brain died eight years ago at the age of 92. He died of aspiration pneumonia and no surviving relatives provided medical or family history information.
The researchers observed the old man's hematoxylin and eosin-stained specimens under a microscope and found extensive spongy changes in the cerebral cortex, vacuoles of different sizes did not tend to merge, and neurons were mostly preserved, while some mild Degree of gliosis. Without frozen tissue or blood samples, researchers would not be able to confirm the presence of mutations in the genes encoding prion proteins.
 Using immunohistochemical staining of the processed specimens, the researchers found that the old man's prion may carry the V180I mutation. Abnormal prion protein (PrPSc) showed only weak signal in the temporal cortex. Because of the small sample size of prions, the researchers could not determine whether the prions in their bodies were contagious.
Using immunohistochemical staining of the processed specimens, the researchers found that the old man's prion may carry the V180I mutation. Abnormal prion protein (PrPSc) showed only weak signal in the temporal cortex. Because of the small sample size of prions, the researchers could not determine whether the prions in their bodies were contagious."It is concerning that there is a possibility of exposure to prions through accidental contact with prion-infected tissue, which puts others at risk of transmission," the researchers said in the communication.
The discovery of prions in cadavers means that all personnel involved in autopsy processing and autopsies need to wear personal protective equipment and follow safety protocols. They need to avoid direct body contact with dead bodies and bodily fluids, as well as avoid injuries such as cuts when examining tissue and taking samples.
Prions are resistant to all standard sterilization measures, and other patients, surgeons, pathologists, and technicians handling infected tissues and instruments are at risk of infection.
To ensure safety, sterilize them by autoclaving at 132°C for 1 hour, or soak them in sodium hydroxide 1 N (normal) or 10% sodium hypochlorite solution for 1 hour.
The global incidence of known prion diseases is estimated at one to two cases per million people per year, the researchers wrote in the newsletter. The incidence of undiagnosed prion diseases, although unknown, is estimated to be as high as one case per 30,000 people.
In this case, it is important to determine whether the etiology is iatrogenic, familial, or sporadic.
Prion diseases are present in many mammals (eg, mink, elk, deer, domesticated cattle and sheep) and can be transmitted between different species through the food chain. There have been previous cases of people at risk of becoming infected after eating beef with bovine spongiform encephalopathy (BSE, or mad cow disease).
Related Posts
0 Comments
Write A Comments