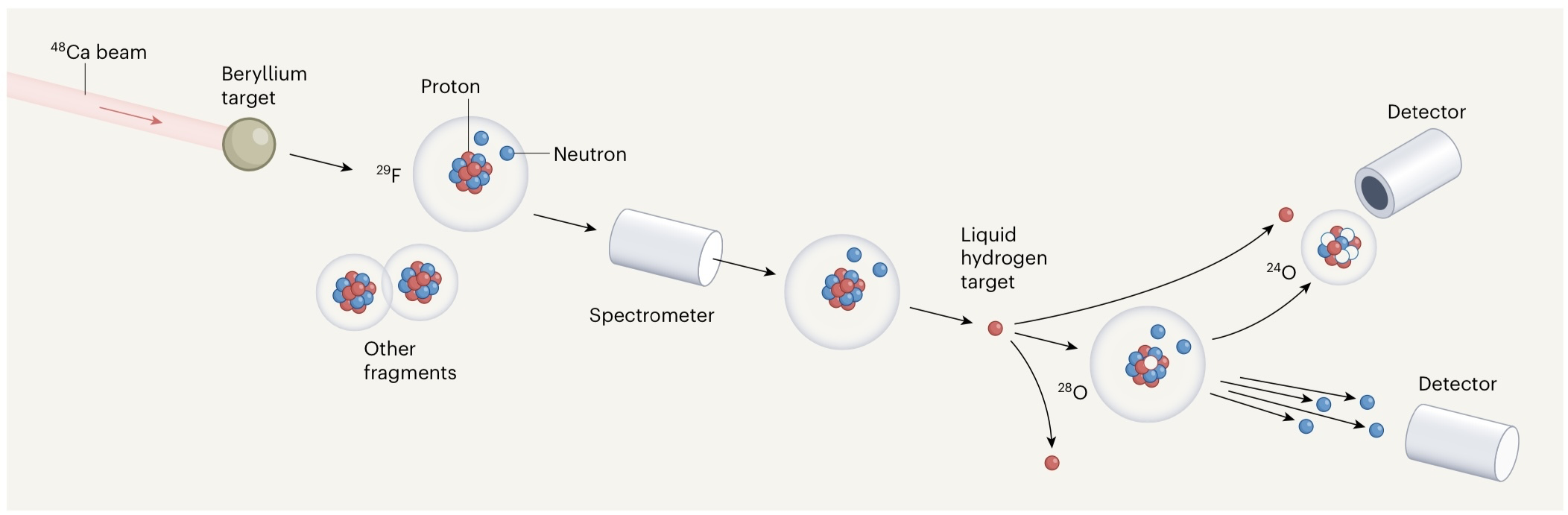
A beam of calcium-48 isotope bombards a beryllium target, which sifts through, "knocking out" protons to produce the heaviest oxygen isotope, oxygen-28.
The world's heaviest oxygen isotope, oxygen-28, has been found for the first time. People's research on it is expected to strictly test the modern theory or model of atomic nucleus structure.
At about 23:00 on August 30, Beijing time, the top international academic journal Nature published a paper stating that extremely neutron-rich oxygen isotopes—oxygen-27 and oxygen-28—were observed for the first time.
The corresponding author of the paper is Yosuke Kondo, assistant professor of the Department of Physics, Tokyo Institute of Technology, Japan. People from Japan, Germany, France, Sweden, the Netherlands, South Korea, Lebanon and other countries formed a joint research team.
"They used the world's most powerful cyclotron to accomplish the challenging feat of finding oxygen-28." Rituparna Kanungo, professor of physics at Saint Mary's University in Canada and scientist at the Canadian Particle Accelerator Center ) stated in his review article.
While the existence of the isotope oxygen-28 has been predicted, no one had previously observed this isotope with its extremely short decay time. It was previously thought that the neutrons and protons in the oxygen-28 nucleus are strongly bound. But experiments now show that it exists in an unbound state.
In the aforementioned research, oxygen-27 and oxygen-28 were artificially created: they excited a high-intensity stable calcium-48 isotope beam to a total energy of about 16 billion electron volts and bombarded a rotating beryllium target. . The collisions split calcium-48 into lighter nuclei, mostly shorter-lived rare isotopes. The debris flies through a spectrometer, which screens out the isotope fluorine-29. The latter has only one more proton than oxygen-28. The fluorine-29 then collides with liquid hydrogen, which "knocks off" a proton to produce oxygen-28.
Kondo Yosuke and others measured the momentum vector of the decay products in the experiment and used these results to deduce the energy spectrum of oxygen-28. They found that the peak of the energy spectrum was quite low, about 0.5 MeV. This finding contradicted all previous theoretical predictions.
According to the observation and calculation results in the experiment, the researchers inferred that neither oxygen-27 nor oxygen-25 could be produced by the decay of oxygen-28. The decay of oxygen-28 proceeds sequentially: first two neutrons are emitted to form an oxygen-26 nucleus, and then two neutrons are emitted to form a bound oxygen-24 nucleus.
The atomic nucleus is considered to be a quantum many-body system composed of protons and neutrons. Since physicist Rutherford proposed that atoms have nuclei in 1911, people have continued to explore the structure of atomic nuclei for more than 110 years.

For his contributions to the theory of atomic nuclei and elementary particles, especially the discovery and application of the basic principles of symmetry, American physicist Eugene Paul Wigner (left) was awarded the Nobel Prize in Physics in 1963; For their discoveries in the core-shell structure, American physicist Maria Goeppert Meyer (middle) and Professor Johannes Hans Daniel Jensen (right) of Heidelberg University in Germany were also awarded that year. Nobel Prize in Physics.
According to information released on the official website of the Nobel Prize, according to modern physics, an atom consists of a nucleus composed of nucleons (protons and neutrons), and electrons distributed in surrounding shells. In 1949, American physicist Maria Goeppert Mayer and Professor Johannes Hans Daniel Jensen of the University of Heidelberg in Germany developed a theoretical model that In this model, nucleons are distributed in shells of different energy levels. In 1963 , Maria Goeppert Meyer and Johannes Hans Daniel Jensen were awarded the Nobel Prize in Physics that year for their discovery of the shell structure of the atomic nucleus, with each receiving a quarter. One bonus.
To put a physical system under extreme conditions is to better understand and gain insight into its organization and structure. In the case of nuclei, one approach is to study isotopes with neutron-to-proton ratios (N/Z) that are very different from those in stable nuclei. Neutron-rich isotopes, especially light isotopes with neutron-to-proton ratios that are significantly different from stable nuclei, provide a rigorous test of modern theoretical models of nuclear structure.
Isotopes are also atoms. Atoms with the same number of protons but different numbers of neutrons are called isotopes. They have almost the same chemical properties but differ in mass and therefore different physical properties. Unstable isotopes are called radioactive isotopes because of decay and radiation.
Oxygen has more than 10 known isotopes, only three of which are naturally occurring, stable isotopes—oxygen-16, oxygen-17, and oxygen-18, and the others are all radioactive.
Oxygen-16 is the most abundant and makes up the vast majority of the oxygen we breathe. There are 8 protons and 8 neutrons in its nucleus.
Oxygen-28 has 8 protons and 20 neutrons in its nucleus.
The researchers believe that the new insights provided by the aforementioned discoveries will deepen our understanding of the structure of atomic nuclei, especially extremely neutron-rich nuclei. Furthermore, the detailed study of multiple neutron correlations and the study of other exotic systems is now possible thanks to the multiple neutron decay spectroscopy technique used in the aforementioned paper.