
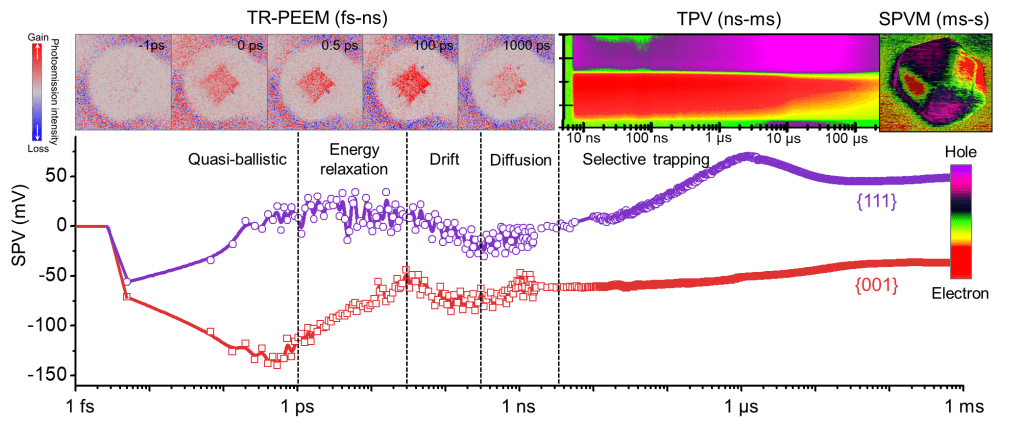
Full spatiotemporal in situ dynamic images of single photocatalytic particles from femtosecond (trillionth of a second) to second photogenerated charge separation process. Source: Photo courtesy of Dalian Institute of Chemistry, Chinese Academy of Sciences
Efficient utilization of solar energy is the scientific "holy grail" of clean energy research.
On October 12, "Nature" published an important advance in solar photocatalysis research online. Through the comprehensive integration of a variety of technologies that can be connected at the spatio-temporal scale, academician Li Can, researcher Fan Fengtao and other researchers from the Dalian Institute of Chemical Physics, Chinese Academy of Sciences have carried out all-time and space detection of the photogenerated charge transfer of photocatalyst nanoparticles, which is the first time in the world. Take" to a full-time image of the evolution of photogenerated charge transfer.
"This study provides a new understanding and research strategy for breaking through the 'bottleneck' of charge separation in photocatalysts for water splitting," emphasized Can Li.
The solar photocatalytic reaction can realize the splitting of water to produce hydrogen and the reduction of carbon dioxide to produce solar fuel, which is expected to provide an important solution for the realization of the "two-carbon" goal, and has attracted worldwide attention.
"Although great efforts have been made in photocatalyst preparation and photocatalytic reaction research in the past half century of photocatalytic research, due to the spatiotemporal complexity of separation, transfer and participation in chemical reactions of photogenerated charges in photocatalytic reactions, People have not been clear about the basic mechanism of this process." Li Can said frankly.
In the photocatalytic process, when light is irradiated on the catalyst, photogenerated charges, that is, photogenerated electrons and holes, are generated inside the catalyst. Photogenerated electrons and holes need to be separated from the interior of the micro- and nano-catalyst particles and transferred to the surface of the catalyst to initiate chemical reactions.
The core scientific challenge of photocatalytic processes lies in how to achieve efficient separation and transport of photogenerated charges. Unraveling the microscopic mechanisms of this process is extremely challenging, as this process spans enormous spatiotemporal scales from femtoseconds to seconds, and from atoms to micrometers.
"Our team has been working on solving this problem for a long time. In this study, we tracked the entire process of the separation and transfer evolution of photogenerated charges in photocatalyst nanoparticles in the entire space-time domain," said Can Li.
To better understand the separation mechanism of photogenerated charges inside the catalyst in the nanosecond range, the researchers used time-resolved photoemission electron microscopy and found that photogenerated electrons can move from one surface to another on a sub-picosecond timescale.
Subsequently, in order to directly observe the transfer process of the photogenerated charges, the researchers performed instantaneous photovoltage analysis and found that as the time scale progressed from nanoseconds to microseconds, holes gradually appeared on the surface of the catalyst containing defects.
"By integrating multiple advanced characterization techniques and theoretical simulations, including time-resolved light emission microscopy, transient surface photovoltage spectroscopy, and surface photovoltage microscopy, etc., like a relay race, electrons and voids are tracked in a photocatalyst particle for the first time. The whole mechanism from the hole to the surface reaction center." Li Can said that the ability to track charge transfer in space and time will greatly promote the understanding of the complex mechanism in the energy conversion process, and provide new ideas and research for rationally designing photocatalysts with better performance. method.
"This is a major breakthrough in basic research. In the future, this achievement is expected to promote the application of solar photocatalytic water splitting to produce solar fuel in real life, make dreams gradually become reality, and provide clean and green energy for our production and life. ” Li Can said.
(Original title "For the first time in the world! My scientist "photographed" a full-time image of the evolution of photogenerated charge transfer")