In 1817, the British physician Dr. James Parkinson published a short treatise on palsy with tremors ("An Essay on the Shaking Palsy"), reporting 6 patients, 3 of which were recorded by his observations on the streets of London, England. This was the first clear description of what came to be known as Parkinson's disease (PD). In 1877, the French doctor Jean-Martin Charcot officially named the disease, and then it received widespread attention.
More than 200 years later, Parkinson's disease has become the second most common neurodegenerative disease after Alzheimer's disease (AD), affecting the health of millions of people. China is also the country with the largest number of Parkinson's disease patients. World Health Organization experts predict that by 2030, China's Parkinson's disease patients will reach 5 million.
"The world is entering an aging society, and our team began to focus on aging-related neurodegenerative diseases about 5 years ago." Professor Feng Guoping, chair professor of the McGovern Institute for Brain Research at the Massachusetts Institute of Technology (MIT) and academician of the American Academy of Arts and Sciences In an interview with The Paper (www.thepaper.cn), the team said that in recent years, Alzheimer's disease, Parkinson's disease and other diseases have become the focus of their research.
On the evening of June 8, Beijing time, the top academic journal "Nature" published online a study jointly completed by MIT, Zhejiang University School of Medicine, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences and other teams, entitled "Targeting thalamic circuits rescues motor and mood deficits in PD mice".
In a mouse model of acute Parkinson's disease, the research team found that chemical genetic manipulation of thalamic parafascicular nucleus (PF) neurons projecting the caudate putamen (CPu) can lead to long-term recovery of mouse movement, while PF→hypothalamic nucleus Optogenetic long-term potentiation (LTP) of (STN) loop synapses restores motor learning behavior. Furthermore, activation of PF neurons projecting the nucleus accumbens (NAc) ameliorates the depression-like phenotype in mice.
Interestingly, the study not only identifies the circuit mechanism of motor and non-motor disorders in Parkinson's mice, but also suggests an exciting therapeutic avenue. "We found that nicotinic acetylcholine receptors (nAChRs) in different circuits can be used as molecular targets to improve part of the phenotype of Parkinson's." The research team believes that targeting the PF thalamic circuit may be a treatment for Parkinson's disease. Effective strategies for movement disorders. Feng Guoping and two postdoctoral fellows in his research group, Zhang Ying and Dheeraj S. Roy, are the corresponding authors of the study. Zhang Ying is also the first author, and Dheeraj S. Roy is the co-first author.
Feng Guoping and two postdoctoral fellows in his research group, Zhang Ying and Dheeraj S. Roy, are the corresponding authors of the study. Zhang Ying is also the first author, and Dheeraj S. Roy is the co-first author.
Feng Guoping received his undergraduate degree from Zhejiang Medical University in 1982, his master degree from Shanghai Second Medical University in 1985, and his Ph.D. degree from the State University of New York at Buffalo in 1995. He later worked as a postdoctoral researcher at Washington University in St. Louis. In 2000, he worked as an assistant professor in the Department of Neurobiology, Duke University Medical Center, and was promoted to associate professor in 2008. In 2010, he joined MIT as a tenured professor of Poitras. His long-term focus is on the study of synapse and circuit functions and the mechanism of neurological disease, and the creation of new technologies for the study of the nervous system.
Parkinson's disease treatment goes beyond motor symptoms
"Three years ago, I saw that a relative next to me started to walk a little slowly, and then I saw that his words became smaller and smaller, and I knew what disease he had when I saw it." Mao Ying, president of Huashan Hospital Affiliated to Fudan University The professor had previously used his relatives suffering from Parkinson's disease as an example to explain the situation of an 85-year-old Parkinson's patient to a reporter from The Paper (www.thepaper.cn).
This symptom is really just a motor symptom that is easily observed in Parkinson's patients. Parkinson's disease is a common neurodegenerative disease in middle-aged and elderly people. The main pathological changes are the progressive degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNc) and the formation of Lewy bodies. Clinical manifestations include motor symptoms such as tremor, muscle rigidity, bradykinesia, and postural balance disorders, as well as non-motor symptoms such as sleep disorders, olfactory disorders, autonomic dysfunction, cognitive and mental disorders.
Epidemiological surveys show that the prevalence of Parkinson's disease in Europe and the United States is 1% over the age of 60, and more than 4% over the age of 80. The prevalence rate of people over 65 years old in China is 1.7%, which is similar to that in European and American countries. As the disease progresses, the motor and nonmotor symptoms of Parkinson's disease can gradually worsen.
Zhang Ying said, "Mobility disorders are the main symptoms of Parkinson's disease, so the current research and treatment of Parkinson's disease focus on motor function, and there has been good progress."
It is mentioned in the "Chinese Parkinson's Disease Treatment Guidelines (Fourth Edition)" that levodopa is currently the standard treatment for Parkinson's disease and the most effective symptomatic drug in the drug treatment of Parkinson's disease. However, in most patients, motor complications, including fluctuating symptoms and dyskinesia, develop with disease progression and long-term levodopa use.
In addition, the main surgical methods are nerve nucleus destruction and deep brain stimulation (DBS). DBS has become the main surgical choice because of its relative non-invasiveness, safety and controllability. The surgical targets mainly include the medial part of the globus pallidus (GPi) and the subthalamic nucleus (STN). Currently, these two targets are considered to have significant curative effects on tremor, rigidity, bradykinesia and dyskinesia, but the subthalamic nucleus-DBS has a significant effect. It is more advantageous to reduce the dose of anti-Parkinson's disease drugs.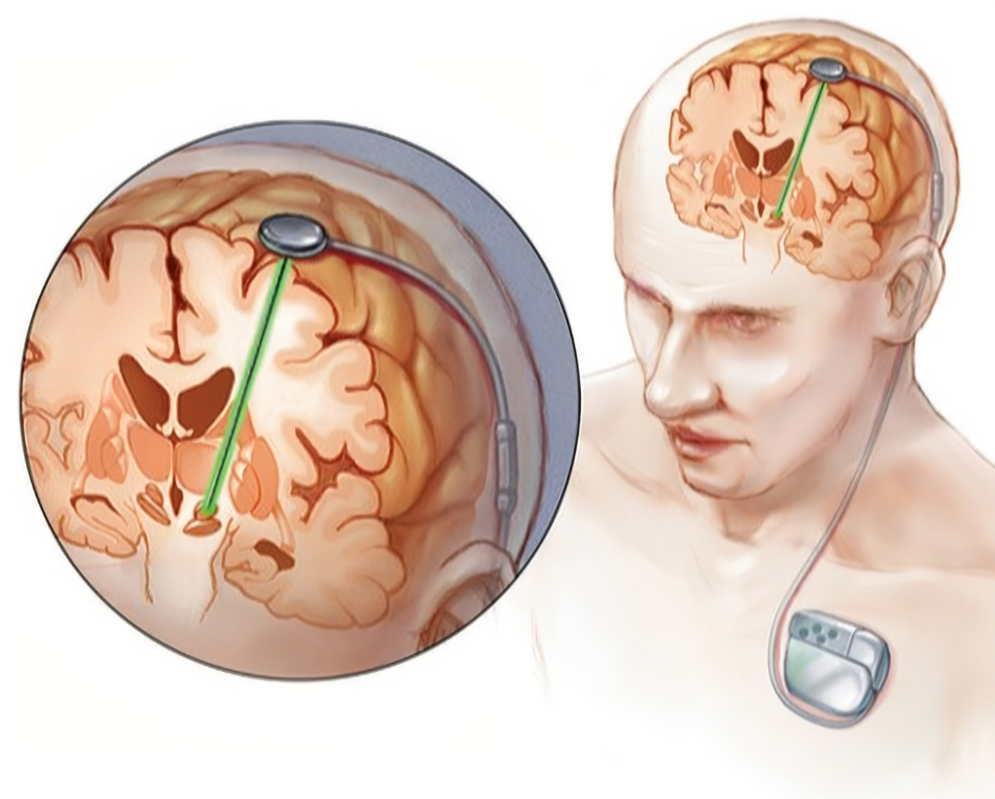 However, the dilemma we face is that, whether it is drugs or surgery, these current treatments can only improve the symptoms, not prevent the development of the disease, let alone cure.
However, the dilemma we face is that, whether it is drugs or surgery, these current treatments can only improve the symptoms, not prevent the development of the disease, let alone cure.
In addition to motor symptoms, the treatment of non-motor symptoms of Parkinson's patients is almost blank. "The development of any therapeutic strategy requires scientists to study the pathogenic mechanisms of these phenotypes, and there are relatively few studies on the non-motor symptoms of Parkinson's disease." Zhang Ying mentioned.
"Parkinson's disease patients also have many non-motor symptoms, such as depression, sleep disturbance, etc. These symptoms, like movement disorders, seriously affect the patient's quality of life." Zhang Ying said that if the patient's motor function is improved at the same time, Improve the mood and sleep quality of patients, "this will be a relatively big progress in the clinical treatment of Parkinson's disease."
The brain is a complex network. One of the long-term research directions of Feng Guoping's team is the function of synapses and circuits and the mechanism of psychiatric diseases. "We hope to provide new ideas for the treatment of aging-related neurodegenerative diseases." Zhang Ying et al. wanted to answer in this study: to determine whether the neural circuit mechanism of motor and non-motor disorders in Parkinson's disease, whether And so on to bring new treatments?
The thalamus and different cell subsets: which symptoms are affected by each?
The thalamus is the main structure of the diencephalon. The human thalamus is two oval gray matter nuclei, each about 5.7 cm long. The thalamus on both sides is connected to a certain extent through the interthalamus adhesion. The thalamus has extensive connections to the basal ganglia (a collective term for a large block of gray matter under the cerebral cortex) and has important contributions to motor behavior. Previous studies and clinical studies have also demonstrated that the subthalamic nucleus-DBS can modulate the pathophysiological changes associated with Parkinson's disease.
It is worth noting that in mammals such as humans, the cerebral neocortex replaces the midbrain and thalamus to take over advanced functions. It is generally believed that the thalamus of higher animals mainly acts as a relay for signal transmission. However, a growing body of research suggests that the role of the thalamus is far more than a "relay station".
Zhang Ying mentioned that this research work started about 5 years ago, and the research team recently published another study on the role of another thalamic nucleus in cognitive decline caused by aging.
In May of this year, Feng Guoping’s team published a study in the Proceedings of the National Academy of Sciences (PNAS) in which Zhang Ying et al. used the latest transgenic mice in the laboratory to specifically regulate different subregions of the anterior thalamus (ANT). , found that the anterior ventral region (AV) specifically mediates the storage of working memory; as mice age, working memory declines, accompanied by a decrease in the excitability of anterior thalamic neurons. The research team used optogenetic methods to increase the activity of this group of neurons, showing that it can significantly improve the working memory function of old mice.
"These advances are all related to a study we published last year exploring shared mechanisms across different diseases. That study pointed to the possibility that the thalamus, a brain region thought to be a switch in the brain, may be involved in more Many unexpected 'non-transit' functions are involved in the pathogenesis of different brain diseases." Zhang Ying said.
Previous studies have shown that the parafascicular nucleus (PF) of the thalamus has a projection relationship with multiple nuclei in the basal ganglia, mainly to the dorsal striatum (i.e., the caudate putamen, CPu), the hypothalamic nucleus (STN), and the ventral ganglia. Lateral striatum (ie, nucleus accumbens NAc) projections. However, the physiological properties of these PF subsets and their circuits have not been extensively studied.
Using anterograde tracing and retrograde tracing techniques, the research team found that PF neurons projecting CPu or STN (PFCPu or PFSTN, respectively) were intermingled in the lateral PF, while PF neurons (PFNAc) projecting NAc were located in the medial PF, suggesting that There are differences in the spatial localization of PF subgroups. In terms of basic electrophysiological properties, PFCPu and PFSTN neurons are similar, in contrast, PFNAc neurons are different, showing the most unique electrophysiological characteristics. Meanwhile, the photo-induced currents of the PF→CPu loop and the PF→NAc loop are larger than those of the PF→STN loop.
The research team believes that these observations suggest that PF neurons projecting to the CPu, STN and NAc belong to distinct subpopulations.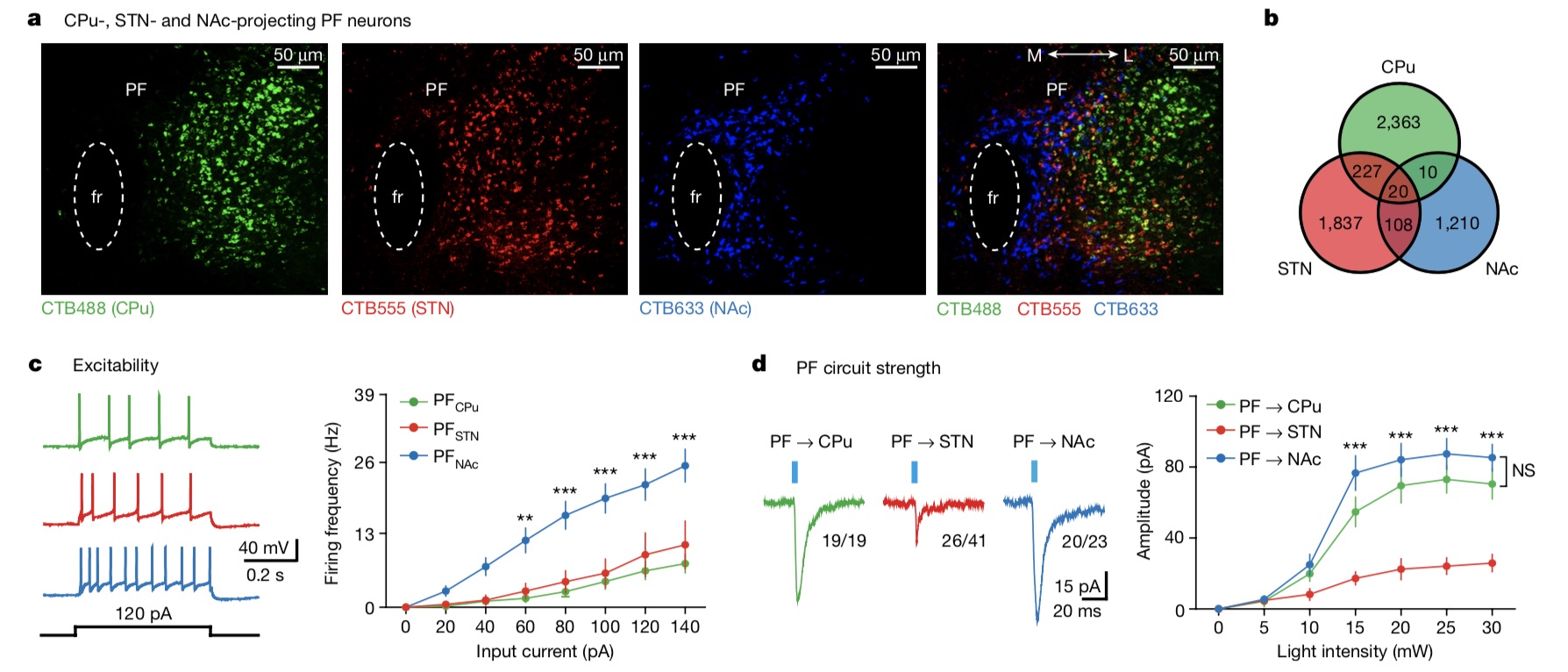
The research team also used the rotarod assay to test whether these PF subsets were associated with motor learning. The rotarod fatigue test is a convenient method to detect motor function in rodents, and can assess the effects of central nervous system disease or damage and drugs on motor coordination function and fatigue by measuring the duration of animals walking on a roller. They found that while PFCPu neurons contribute to general motor activity, PFSTN neurons are critical for motor learning, revealing distinct functional roles for these two PF subpopulations.
The study further mentions that it is widely believed that the major cell type of the STN expresses the glutamate marker VGLUT2 (encoded by Slc17a6). However, the STN also contains a subpopulation positive for parvalbumin (PV, encoded by the Pvalb gene), which remains to be studied. They found experimentally that there were more PF neurons projecting to Pvalb+ STN neurons than to Vglut2+ STN neurons. And further supported by inhibition separately, only the mice in the Pvalb+ inhibition group were impaired in motor learning, a new step supporting the role of Pvalb+ STN neurons in motor learning.
Importantly, the research team's mechanistic discovery of STN in rodents may be conserved in higher species, such as non-human primates, and even in humans.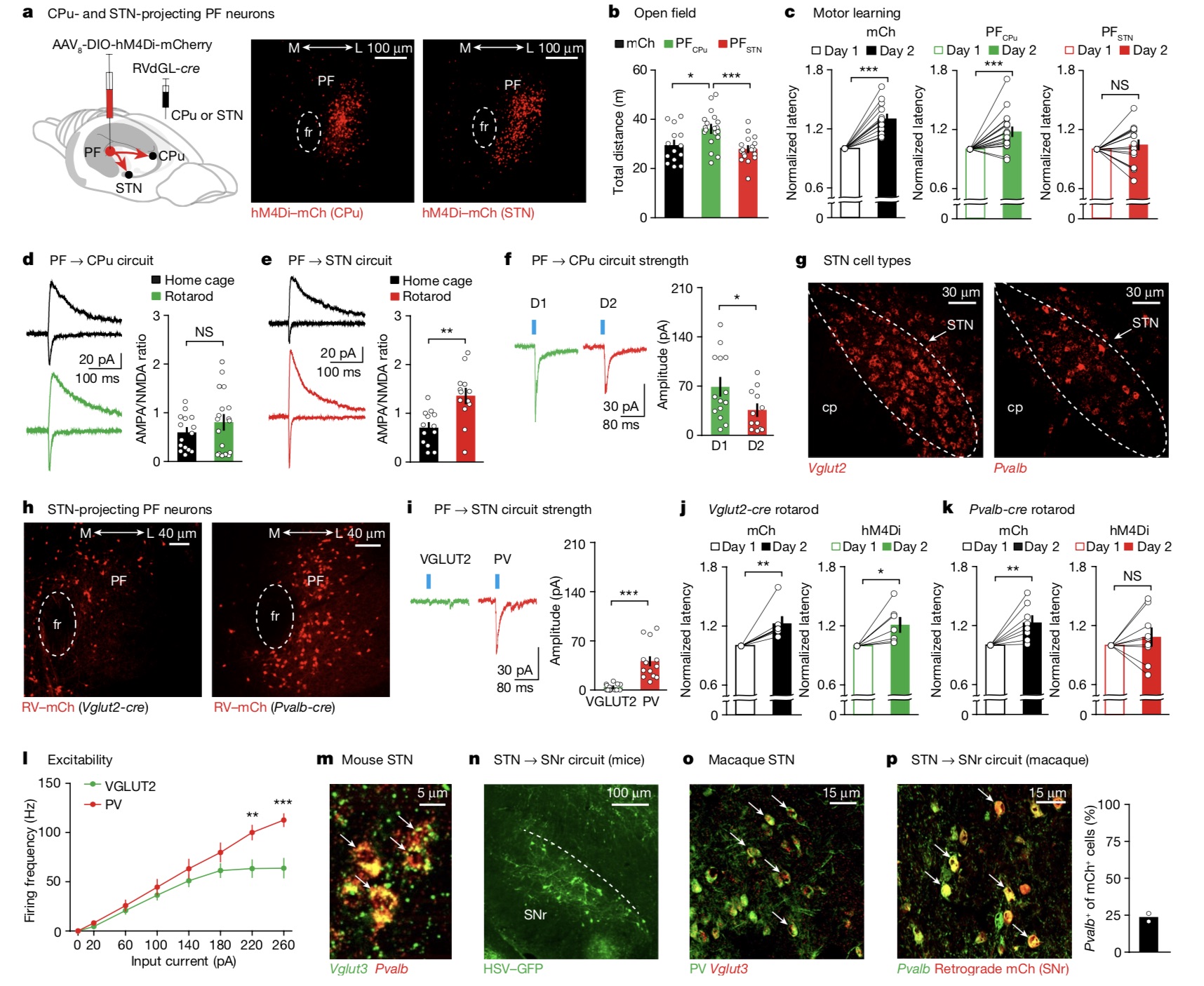
The results showed that compared with control mice, PFNAc neuron-suppressed mice decreased by 14.75% in the sugar water preference test, increased by 38.28% in the forced swim test, and increased by 39.83% in the tail suspension test. This further supports the role of PFNAc neurons in reward processing, revealing a critical role for PFNAc subsets in non-motor behavior.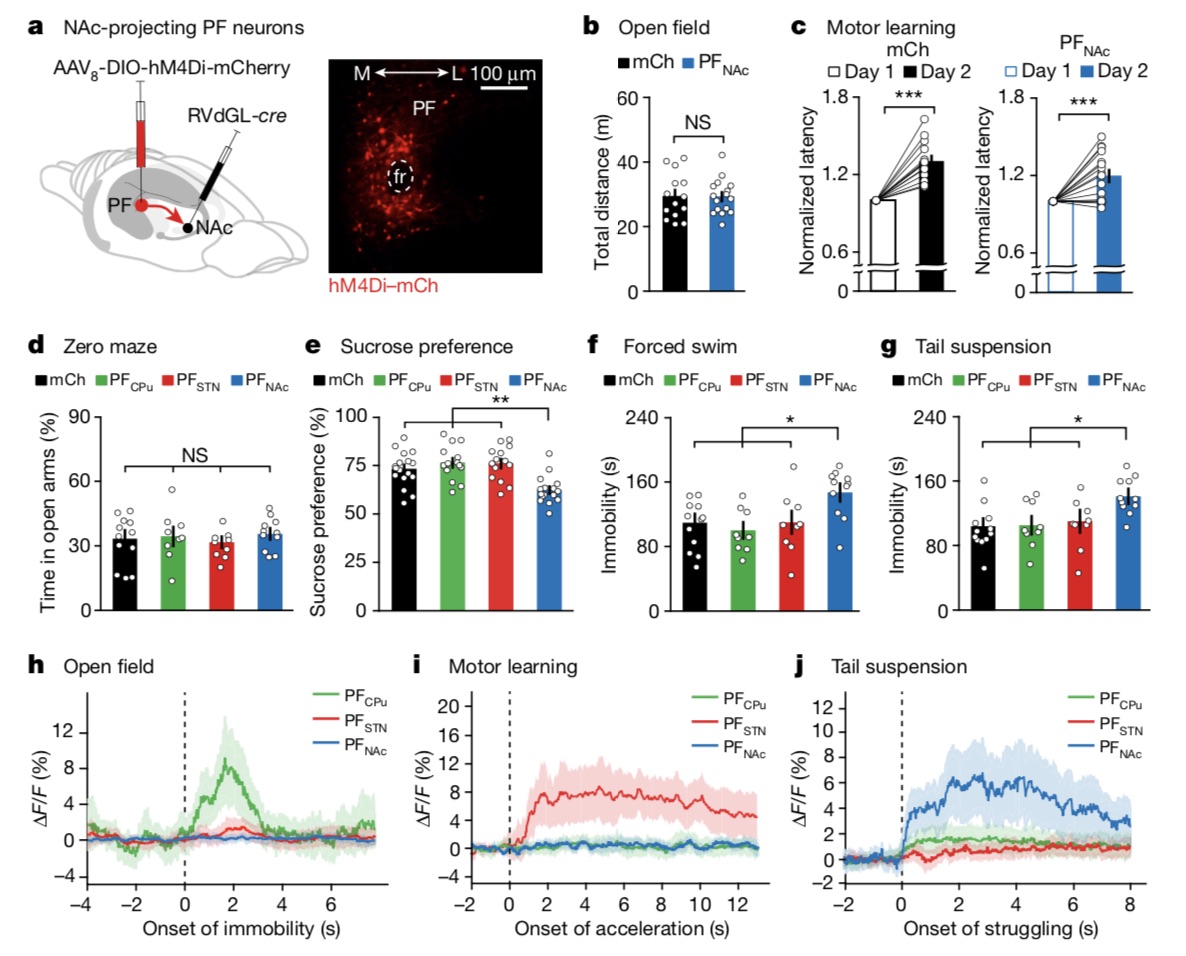
Zhang Ying emphasized, "We now understand that these different cell subsets play different roles in the pathogenesis of various symptoms of Parkinson's disease. If we do not distinguish these subsets, but generally regulate the entire thalamus, it will be very It is difficult to see consistent effects in improving the symptoms of the disease."
Molecular targets are found, but multiple key issues still need to be solved before going to the clinic
What new hints can these basic research results bring to the clinical treatment of Parkinson's disease?
The research team continued to explore this answer and established a mouse model of acute Parkinsonism (PD mice) by bilaterally injecting 6-hydroxydopamine into the substantia nigra pars compacta (SNc).
Similar to Parkinson's disease patients, Parkinson's disease mouse models have impairments in both motor and motor learning. The study found that with regard to motor recovery in PD model mice, acute inhibition of PFCPu neurons immediately improved behavioral performance. However, they found that the improvement did not last long, but to their surprise, prolonged inhibition of PFCPu neurons resulted in long-term motor recovery in PD model mice for as long as 10 days.
It is worth mentioning that previous studies have shown that the activation of the PF→STN loop can increase movement, and Zhang Ying et al. found in this study that the PF→STN loop is necessary for motor learning, and this loop was also impaired in PD model mice. Taken together, these observations suggest that activation of the PF→STN loop may alleviate motor and motor learning impairments in Parkinson's, the research team believes.
In addition to the motor phenotype, depression-like symptoms were also observed in PD mice. Using a minimally invasive optogenetic approach to modulate PFNAc neurons, the research team demonstrated that activating this specific subset of PF in PD mice is sufficient to alleviate depression-like behaviors.
However, the team noted that women are about twice as likely to develop depression as men, and their findings were based only on male mice, and further research is needed to determine whether these circuit manipulations are equally effective in female mice.
The research team further tested whether a similar effect could be achieved with molecular targets? They ultimately screened to find that nicotinic acetylcholine receptors (nAChRs), which target distinct PF circuits, offer a potentially exciting therapeutic approach to alleviating motor and non-motor impairments in PD.
Overall, this study found that nAChRs in different loops can serve as molecular targets to improve some Parkinson's phenotypes, Zhang Ying said.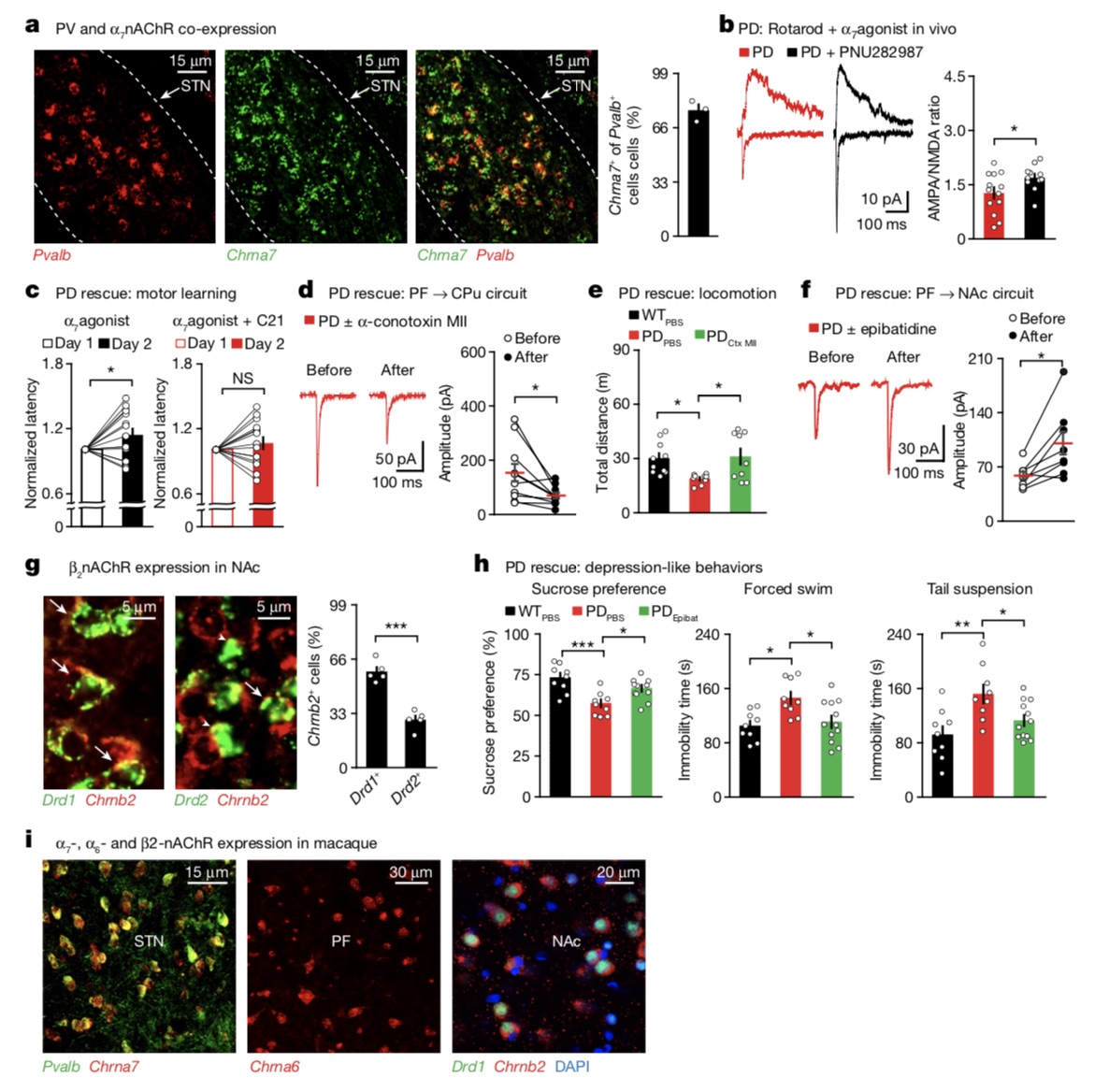
The second problem mentioned by Zhang Ying is that this study uses a mouse model for systematic research, "In the next time, we will verify the pathogenic mechanisms found in mice and the molecular targets in primates. Is it applicable in the class."
Interestingly, this study also shows us the importance of new technology applications in the field of neuroscience. Feng Guoping's team has also long been committed to creating new technologies for nervous system research. In this study, the team's previously developed SOUL protein-based optogenetics technology was also used.
The so-called optogenetics is a technology that combines optics and genetics. With this technology, researchers can precisely control the growth of specific types of neurons in the brain, spinal cord, and peripheral nerves of living animals and even freely moving animals. Activity. In 2010, optogenetics was selected as the method of the year by Nature Methods, and in the same year, it was recognized by Science as one of the breakthroughs of the past decade.
"In recent years, the development of optogenetics has provided new methods for the study of brain disease mechanisms; at the same time, gene editing and the development of small molecules that cross the blood-brain barrier have also made scientific research a step forward in the direction of clinical translation." The team told The Paper that the progress of scientific research is inseparable from the development of technology, and the emergence of important technologies can bring about a revolution in the entire research field.
More than 200 years later, Parkinson's disease has become the second most common neurodegenerative disease after Alzheimer's disease (AD), affecting the health of millions of people. China is also the country with the largest number of Parkinson's disease patients. World Health Organization experts predict that by 2030, China's Parkinson's disease patients will reach 5 million.
"The world is entering an aging society, and our team began to focus on aging-related neurodegenerative diseases about 5 years ago." Professor Feng Guoping, chair professor of the McGovern Institute for Brain Research at the Massachusetts Institute of Technology (MIT) and academician of the American Academy of Arts and Sciences In an interview with The Paper (www.thepaper.cn), the team said that in recent years, Alzheimer's disease, Parkinson's disease and other diseases have become the focus of their research.
On the evening of June 8, Beijing time, the top academic journal "Nature" published online a study jointly completed by MIT, Zhejiang University School of Medicine, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences and other teams, entitled "Targeting thalamic circuits rescues motor and mood deficits in PD mice".
In a mouse model of acute Parkinson's disease, the research team found that chemical genetic manipulation of thalamic parafascicular nucleus (PF) neurons projecting the caudate putamen (CPu) can lead to long-term recovery of mouse movement, while PF→hypothalamic nucleus Optogenetic long-term potentiation (LTP) of (STN) loop synapses restores motor learning behavior. Furthermore, activation of PF neurons projecting the nucleus accumbens (NAc) ameliorates the depression-like phenotype in mice.
Interestingly, the study not only identifies the circuit mechanism of motor and non-motor disorders in Parkinson's mice, but also suggests an exciting therapeutic avenue. "We found that nicotinic acetylcholine receptors (nAChRs) in different circuits can be used as molecular targets to improve part of the phenotype of Parkinson's." The research team believes that targeting the PF thalamic circuit may be a treatment for Parkinson's disease. Effective strategies for movement disorders.
 Feng Guoping and two postdoctoral fellows in his research group, Zhang Ying and Dheeraj S. Roy, are the corresponding authors of the study. Zhang Ying is also the first author, and Dheeraj S. Roy is the co-first author.
Feng Guoping and two postdoctoral fellows in his research group, Zhang Ying and Dheeraj S. Roy, are the corresponding authors of the study. Zhang Ying is also the first author, and Dheeraj S. Roy is the co-first author.Feng Guoping received his undergraduate degree from Zhejiang Medical University in 1982, his master degree from Shanghai Second Medical University in 1985, and his Ph.D. degree from the State University of New York at Buffalo in 1995. He later worked as a postdoctoral researcher at Washington University in St. Louis. In 2000, he worked as an assistant professor in the Department of Neurobiology, Duke University Medical Center, and was promoted to associate professor in 2008. In 2010, he joined MIT as a tenured professor of Poitras. His long-term focus is on the study of synapse and circuit functions and the mechanism of neurological disease, and the creation of new technologies for the study of the nervous system.
Parkinson's disease treatment goes beyond motor symptoms
"Three years ago, I saw that a relative next to me started to walk a little slowly, and then I saw that his words became smaller and smaller, and I knew what disease he had when I saw it." Mao Ying, president of Huashan Hospital Affiliated to Fudan University The professor had previously used his relatives suffering from Parkinson's disease as an example to explain the situation of an 85-year-old Parkinson's patient to a reporter from The Paper (www.thepaper.cn).
This symptom is really just a motor symptom that is easily observed in Parkinson's patients. Parkinson's disease is a common neurodegenerative disease in middle-aged and elderly people. The main pathological changes are the progressive degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNc) and the formation of Lewy bodies. Clinical manifestations include motor symptoms such as tremor, muscle rigidity, bradykinesia, and postural balance disorders, as well as non-motor symptoms such as sleep disorders, olfactory disorders, autonomic dysfunction, cognitive and mental disorders.
Epidemiological surveys show that the prevalence of Parkinson's disease in Europe and the United States is 1% over the age of 60, and more than 4% over the age of 80. The prevalence rate of people over 65 years old in China is 1.7%, which is similar to that in European and American countries. As the disease progresses, the motor and nonmotor symptoms of Parkinson's disease can gradually worsen.
Zhang Ying said, "Mobility disorders are the main symptoms of Parkinson's disease, so the current research and treatment of Parkinson's disease focus on motor function, and there has been good progress."
It is mentioned in the "Chinese Parkinson's Disease Treatment Guidelines (Fourth Edition)" that levodopa is currently the standard treatment for Parkinson's disease and the most effective symptomatic drug in the drug treatment of Parkinson's disease. However, in most patients, motor complications, including fluctuating symptoms and dyskinesia, develop with disease progression and long-term levodopa use.
In addition, the main surgical methods are nerve nucleus destruction and deep brain stimulation (DBS). DBS has become the main surgical choice because of its relative non-invasiveness, safety and controllability. The surgical targets mainly include the medial part of the globus pallidus (GPi) and the subthalamic nucleus (STN). Currently, these two targets are considered to have significant curative effects on tremor, rigidity, bradykinesia and dyskinesia, but the subthalamic nucleus-DBS has a significant effect. It is more advantageous to reduce the dose of anti-Parkinson's disease drugs.
 However, the dilemma we face is that, whether it is drugs or surgery, these current treatments can only improve the symptoms, not prevent the development of the disease, let alone cure.
However, the dilemma we face is that, whether it is drugs or surgery, these current treatments can only improve the symptoms, not prevent the development of the disease, let alone cure.In addition to motor symptoms, the treatment of non-motor symptoms of Parkinson's patients is almost blank. "The development of any therapeutic strategy requires scientists to study the pathogenic mechanisms of these phenotypes, and there are relatively few studies on the non-motor symptoms of Parkinson's disease." Zhang Ying mentioned.
"Parkinson's disease patients also have many non-motor symptoms, such as depression, sleep disturbance, etc. These symptoms, like movement disorders, seriously affect the patient's quality of life." Zhang Ying said that if the patient's motor function is improved at the same time, Improve the mood and sleep quality of patients, "this will be a relatively big progress in the clinical treatment of Parkinson's disease."
The brain is a complex network. One of the long-term research directions of Feng Guoping's team is the function of synapses and circuits and the mechanism of psychiatric diseases. "We hope to provide new ideas for the treatment of aging-related neurodegenerative diseases." Zhang Ying et al. wanted to answer in this study: to determine whether the neural circuit mechanism of motor and non-motor disorders in Parkinson's disease, whether And so on to bring new treatments?
The thalamus and different cell subsets: which symptoms are affected by each?
The thalamus is the main structure of the diencephalon. The human thalamus is two oval gray matter nuclei, each about 5.7 cm long. The thalamus on both sides is connected to a certain extent through the interthalamus adhesion. The thalamus has extensive connections to the basal ganglia (a collective term for a large block of gray matter under the cerebral cortex) and has important contributions to motor behavior. Previous studies and clinical studies have also demonstrated that the subthalamic nucleus-DBS can modulate the pathophysiological changes associated with Parkinson's disease.
It is worth noting that in mammals such as humans, the cerebral neocortex replaces the midbrain and thalamus to take over advanced functions. It is generally believed that the thalamus of higher animals mainly acts as a relay for signal transmission. However, a growing body of research suggests that the role of the thalamus is far more than a "relay station".
Zhang Ying mentioned that this research work started about 5 years ago, and the research team recently published another study on the role of another thalamic nucleus in cognitive decline caused by aging.
In May of this year, Feng Guoping’s team published a study in the Proceedings of the National Academy of Sciences (PNAS) in which Zhang Ying et al. used the latest transgenic mice in the laboratory to specifically regulate different subregions of the anterior thalamus (ANT). , found that the anterior ventral region (AV) specifically mediates the storage of working memory; as mice age, working memory declines, accompanied by a decrease in the excitability of anterior thalamic neurons. The research team used optogenetic methods to increase the activity of this group of neurons, showing that it can significantly improve the working memory function of old mice.
"These advances are all related to a study we published last year exploring shared mechanisms across different diseases. That study pointed to the possibility that the thalamus, a brain region thought to be a switch in the brain, may be involved in more Many unexpected 'non-transit' functions are involved in the pathogenesis of different brain diseases." Zhang Ying said.
Previous studies have shown that the parafascicular nucleus (PF) of the thalamus has a projection relationship with multiple nuclei in the basal ganglia, mainly to the dorsal striatum (i.e., the caudate putamen, CPu), the hypothalamic nucleus (STN), and the ventral ganglia. Lateral striatum (ie, nucleus accumbens NAc) projections. However, the physiological properties of these PF subsets and their circuits have not been extensively studied.
Using anterograde tracing and retrograde tracing techniques, the research team found that PF neurons projecting CPu or STN (PFCPu or PFSTN, respectively) were intermingled in the lateral PF, while PF neurons (PFNAc) projecting NAc were located in the medial PF, suggesting that There are differences in the spatial localization of PF subgroups. In terms of basic electrophysiological properties, PFCPu and PFSTN neurons are similar, in contrast, PFNAc neurons are different, showing the most unique electrophysiological characteristics. Meanwhile, the photo-induced currents of the PF→CPu loop and the PF→NAc loop are larger than those of the PF→STN loop.
The research team believes that these observations suggest that PF neurons projecting to the CPu, STN and NAc belong to distinct subpopulations.

There are three distinct subsets of specific projections in the parafascicular nucleus (PF) of the thalamus.
Next, the research team investigated the function of different PF subsets. While PFSTN neuron inhibition had no effect on mouse motility, PFCPu neuron inhibition resulted in a significant increase in motility, a 22.81% increase compared to control mice. The paper cited a previous study in which the team used chemical genetic techniques to suppress the entire PF, but no significant changes were observed. They argue that these different trials suggest that manipulation of PF-specific projections may be more effective in revealing specific behavioral contributions.The research team also used the rotarod assay to test whether these PF subsets were associated with motor learning. The rotarod fatigue test is a convenient method to detect motor function in rodents, and can assess the effects of central nervous system disease or damage and drugs on motor coordination function and fatigue by measuring the duration of animals walking on a roller. They found that while PFCPu neurons contribute to general motor activity, PFSTN neurons are critical for motor learning, revealing distinct functional roles for these two PF subpopulations.
The study further mentions that it is widely believed that the major cell type of the STN expresses the glutamate marker VGLUT2 (encoded by Slc17a6). However, the STN also contains a subpopulation positive for parvalbumin (PV, encoded by the Pvalb gene), which remains to be studied. They found experimentally that there were more PF neurons projecting to Pvalb+ STN neurons than to Vglut2+ STN neurons. And further supported by inhibition separately, only the mice in the Pvalb+ inhibition group were impaired in motor learning, a new step supporting the role of Pvalb+ STN neurons in motor learning.
Importantly, the research team's mechanistic discovery of STN in rodents may be conserved in higher species, such as non-human primates, and even in humans.

PF neurons projecting CPu and STN mediate distinct motor behaviors.
Additionally, although the research team used chemogenetic methods to inhibit PFNAc neurons and found that this subpopulation did not play a critical role in exercise testing, however, given the critical role of NAc in anxiety and depression, they then tested elevated levels of anxiety by testing The zero-maze test, along with three commonly used tests to detect depression-like states, the sugar water preference test, the forced swim test, and the tail suspension test, were tested in PFNAc-inhibited mice.The results showed that compared with control mice, PFNAc neuron-suppressed mice decreased by 14.75% in the sugar water preference test, increased by 38.28% in the forced swim test, and increased by 39.83% in the tail suspension test. This further supports the role of PFNAc neurons in reward processing, revealing a critical role for PFNAc subsets in non-motor behavior.

PF neurons projecting the NAc mediate depression-like behavior.
It should be pointed out that, due to the lack of specific technical means, most of the previous studies were unable to distinguish each thalamic brain area, and there were very few studies on different cell subsets in a single thalamic brain area.Zhang Ying emphasized, "We now understand that these different cell subsets play different roles in the pathogenesis of various symptoms of Parkinson's disease. If we do not distinguish these subsets, but generally regulate the entire thalamus, it will be very It is difficult to see consistent effects in improving the symptoms of the disease."
Molecular targets are found, but multiple key issues still need to be solved before going to the clinic
What new hints can these basic research results bring to the clinical treatment of Parkinson's disease?
The research team continued to explore this answer and established a mouse model of acute Parkinsonism (PD mice) by bilaterally injecting 6-hydroxydopamine into the substantia nigra pars compacta (SNc).
Similar to Parkinson's disease patients, Parkinson's disease mouse models have impairments in both motor and motor learning. The study found that with regard to motor recovery in PD model mice, acute inhibition of PFCPu neurons immediately improved behavioral performance. However, they found that the improvement did not last long, but to their surprise, prolonged inhibition of PFCPu neurons resulted in long-term motor recovery in PD model mice for as long as 10 days.
It is worth mentioning that previous studies have shown that the activation of the PF→STN loop can increase movement, and Zhang Ying et al. found in this study that the PF→STN loop is necessary for motor learning, and this loop was also impaired in PD model mice. Taken together, these observations suggest that activation of the PF→STN loop may alleviate motor and motor learning impairments in Parkinson's, the research team believes.
In addition to the motor phenotype, depression-like symptoms were also observed in PD mice. Using a minimally invasive optogenetic approach to modulate PFNAc neurons, the research team demonstrated that activating this specific subset of PF in PD mice is sufficient to alleviate depression-like behaviors.
However, the team noted that women are about twice as likely to develop depression as men, and their findings were based only on male mice, and further research is needed to determine whether these circuit manipulations are equally effective in female mice.
The research team further tested whether a similar effect could be achieved with molecular targets? They ultimately screened to find that nicotinic acetylcholine receptors (nAChRs), which target distinct PF circuits, offer a potentially exciting therapeutic approach to alleviating motor and non-motor impairments in PD.
Overall, this study found that nAChRs in different loops can serve as molecular targets to improve some Parkinson's phenotypes, Zhang Ying said.

nAChRs targeting different loops can improve Parkinson's phenotype.
However, any basic research needs to go through a very long process before it finally has a chance to go to the clinic. "The first problem is that we are using agonists or antagonists of these targets in our study. We all know that these receptors are widely expressed in the brain, and the use of these agonists or antagonists is bound to cause potential side effects. How? Specifically modulating molecular targets in targeted brain regions in a non-invasive way is the problem we are solving."The second problem mentioned by Zhang Ying is that this study uses a mouse model for systematic research, "In the next time, we will verify the pathogenic mechanisms found in mice and the molecular targets in primates. Is it applicable in the class."
Interestingly, this study also shows us the importance of new technology applications in the field of neuroscience. Feng Guoping's team has also long been committed to creating new technologies for nervous system research. In this study, the team's previously developed SOUL protein-based optogenetics technology was also used.
The so-called optogenetics is a technology that combines optics and genetics. With this technology, researchers can precisely control the growth of specific types of neurons in the brain, spinal cord, and peripheral nerves of living animals and even freely moving animals. Activity. In 2010, optogenetics was selected as the method of the year by Nature Methods, and in the same year, it was recognized by Science as one of the breakthroughs of the past decade.
"In recent years, the development of optogenetics has provided new methods for the study of brain disease mechanisms; at the same time, gene editing and the development of small molecules that cross the blood-brain barrier have also made scientific research a step forward in the direction of clinical translation." The team told The Paper that the progress of scientific research is inseparable from the development of technology, and the emergence of important technologies can bring about a revolution in the entire research field.



Comments